Balancing chemical equations | Chemical reactions | High school chemistry | Khan Academy
TLDRThe script offers a clear explanation of balancing chemical equations, a common point of confusion in chemistry. It begins by defining a chemical equation and illustrating the need for balance using the reaction between aluminum and dioxygen to form aluminum oxide. The tutorial demonstrates the process of balancing by adjusting coefficients to ensure equal numbers of each atom on both sides of the equation, emphasizing the importance of whole number molecules and avoiding fractions. The example concludes with a balanced equation, highlighting the methodical approach to achieving balance.
Takeaways
- 🔬 Balancing chemical equations is an essential concept in chemistry that ensures the same number of atoms for each element is present on both sides of the reaction.
- 📚 A chemical equation describes a reaction, such as the reaction between aluminum and oxygen to form aluminum oxide.
- 🧐 The initial observation in balancing is to identify the discrepancies in the number of atoms for each element between the reactants and products.
- 🤔 The process of balancing involves adjusting the coefficients in front of the chemical formulas to ensure equal numbers of atoms for each element on both sides of the equation.
- 📈 An example given in the script is the reaction of aluminum with dioxygen to form aluminum oxide, where initially, the number of aluminum and oxygen atoms are not balanced.
- 🔍 To balance aluminum, the script suggests doubling the number of aluminum atoms on the side with only one aluminum to match the two aluminum atoms in the aluminum oxide.
- ⚖️ For balancing oxygen, the script demonstrates the process of multiplying by a whole number to avoid fractions, resulting in whole number molecules on both sides of the equation.
- 📝 The convention in chemistry is to use whole number coefficients, avoiding the concept of half or fractional molecules.
- 🔄 The script illustrates the method of multiplying all molecules by the same number to eliminate fractions and achieve a balanced equation.
- 📉 The final step is to verify that the number of atoms for each element is equal on both sides of the equation, confirming the balance.
- 🎨 The script uses color coding to distinguish between the steps and the final balanced equation, aiding in visual clarity and understanding.
Q & A
What is a chemical equation?
-A chemical equation is a representation of a chemical reaction that shows the reactants and products along with their respective quantities.
Why is balancing chemical equations important?
-Balancing chemical equations is important to ensure that the number of atoms for each element is the same on both sides of the equation, adhering to the law of conservation of mass.
What is the initial imbalance in the given example of aluminum reacting with dioxygen?
-The initial imbalance is that there is one aluminum atom and two oxygen atoms on the left side, while on the right side, there are two aluminum atoms and three oxygen atoms in the aluminum oxide molecule.
How can you start balancing the aluminum atoms in the example equation?
-You can start by placing a coefficient of 2 in front of the aluminum on the left side to match the two aluminum atoms on the right side.
What is the issue with the oxygen atoms after initially balancing the aluminum?
-After initially balancing the aluminum, the issue with the oxygen atoms is that there are two oxygen atoms on the left side (as part of a dioxygen molecule) and three oxygen atoms on the right side in the aluminum oxide molecule.
Why can't we have 1.5 molecules in a chemical equation?
-In chemical equations, we cannot have 1.5 molecules because it is not chemically meaningful to have a fraction of a molecule; we must have whole numbers of molecules.
How can you eliminate the fraction when balancing the oxygen atoms?
-You can eliminate the fraction by multiplying both sides of the equation by the same whole number that will convert the fraction into a whole number.
What is the final balanced chemical equation for the reaction between aluminum and dioxygen?
-The final balanced chemical equation is 4Al + 3O2 → 2Al2O3, which shows four aluminum atoms and six oxygen atoms on both sides.
What is the significance of using whole number molecules in chemical equations?
-Using whole number molecules in chemical equations ensures that the reaction is represented in a way that is chemically feasible and adheres to the conservation of mass.
Can you provide a step-by-step process of how the example equation was balanced?
-First, balance the aluminum by doubling it on the reactant side. Then, adjust the oxygen by multiplying the dioxygen molecule by 1.5, but to avoid fractions, multiply everything by 2 to get whole numbers, resulting in 4Al + 3O2 → 2Al2O3.
What is the law of conservation of mass and how does it relate to balancing chemical equations?
-The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. It relates to balancing chemical equations by ensuring that the number of atoms of each element is the same on both sides of the equation.
Outlines
🧪 Understanding Chemical Equations and the Balancing Process
This paragraph introduces the concept of balancing chemical equations, a fundamental yet often confusing topic in chemistry. It explains what a chemical equation is and how it describes a reaction, using the example of aluminum reacting with dioxygen to form aluminum oxide. The paragraph emphasizes the importance of having an equal number of each type of atom on both sides of the equation, highlighting the initial imbalance in the given example. It then explores the process of balancing by adjusting the coefficients in front of the chemical formulas to ensure that the number of aluminum and oxygen atoms is the same on both sides of the equation.
Mindmap
Keywords
💡Chemical Equation
💡Balancing
💡Reactants
💡Products
💡Aluminum
💡Dioxygen
💡Aluminum Oxide
💡Conservation of Mass
💡Coefficients
💡Molecules
💡Whole Number Molecules
Highlights
Balancing chemical equations is a fundamental concept in chemistry that can be confusing but is manageable with careful and methodical approach.
A chemical equation describes a reaction, such as the reaction between aluminum and oxygen to form aluminum oxide.
The initial equation shows an imbalance in the number of aluminum and oxygen atoms on both sides.
Balancing involves ensuring the same number of each type of atom on both sides of the equation.
Initially, there is one aluminum atom on the left and two on the right, indicating a need for balance.
Similarly, the oxygen atoms are imbalanced with two on the left and three in the aluminum oxide molecule on the right.
A simple approach to balance aluminum is to double the number on the left side, resulting in two aluminum atoms on both sides.
For oxygen, multiplying by 1.5 could theoretically balance the atoms, but this is not conventional in chemistry.
Chemistry prefers whole number molecules, avoiding fractions or decimals in chemical equations.
Multiplying both sides of the equation by a whole number can eliminate fractions and achieve balance.
By multiplying all molecules by two, the equation can be balanced with four aluminum atoms and six oxygen atoms on both sides.
The final balanced chemical equation is Al + 3O2 → 2Al2O3, reflecting the correct stoichiometry.
The process of balancing chemical equations is akin to solving algebraic equations, emphasizing the importance of whole number coefficients.
The art of balancing chemical equations requires an understanding of both the reactants and the products in a chemical reaction.
Balancing chemical equations is not only a scientific necessity but also a fundamental skill in understanding chemical reactions.
The final balanced equation ensures that the law of conservation of mass is adhered to, reflecting that matter is neither created nor destroyed in a chemical reaction.
The methodical approach to balancing chemical equations can be applied to various types of chemical reactions, making it a versatile skill in chemistry.
Understanding the process of balancing chemical equations can help in predicting the outcomes of chemical reactions and in designing experiments.
Transcripts
Browse More Related Video

01 - Introduction to the Algebraic Method for Balancing Chemical Equations

Balancing Chemical Equations

GCSE Chemistry - Balancing Chemical Equations #4

Balancing another combustion reaction | Chemical reactions | High school chemistry | Khan Academy

Practice Problem: Balancing Equations
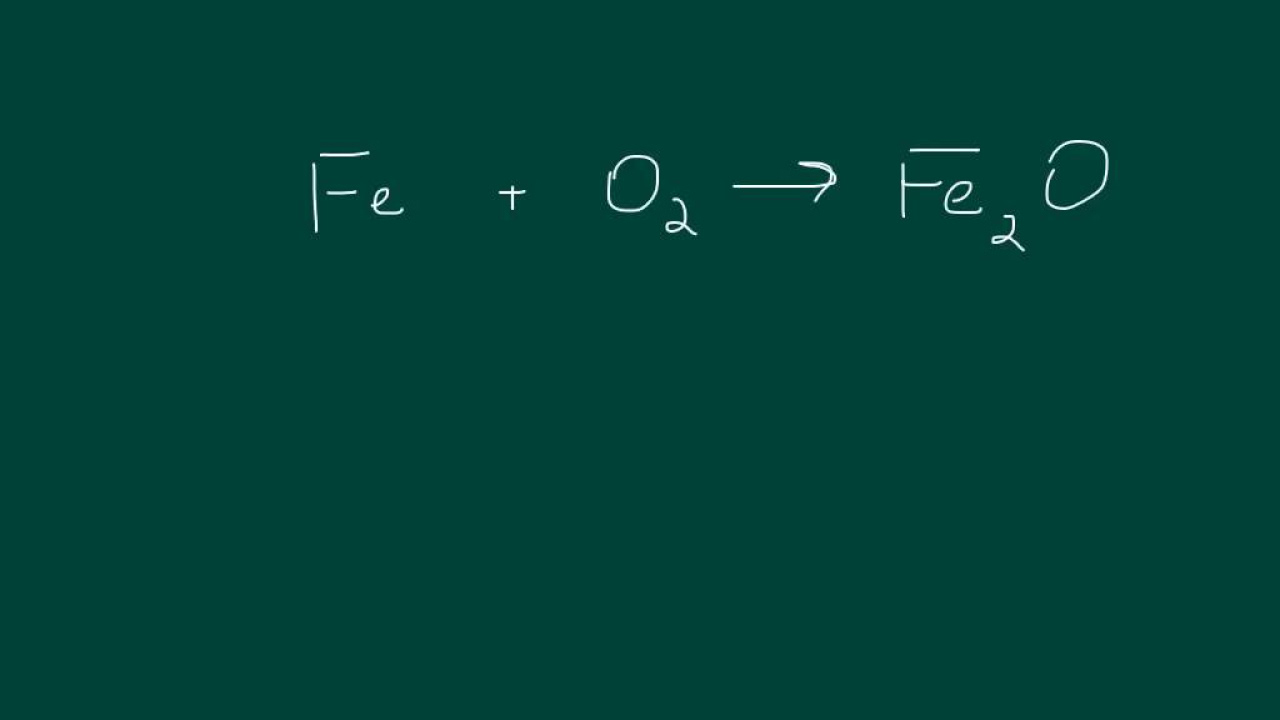
Synthesis Reactions
5.0 / 5 (0 votes)
Thanks for rating: