Practice Problem: Balancing Equations
TLDRThe video script provides a detailed walkthrough on how to balance chemical equations. It begins with a reaction involving CaCl2 and Na3PO4, illustrating the process of creating a balance by adjusting coefficients to ensure equal numbers of each element on both sides of the equation. The script then moves on to a combustion reaction of C2H6O with O2, emphasizing the strategy of balancing elements one by one, with a tip to balance elements in their simplest form last for greater flexibility. Following this, the script tackles the synthesis of C8H18 from CO and H2, highlighting the importance of balancing carbon first due to its presence in multiple molecules. Lastly, the decomposition of NH4NO3 is discussed, where the script demonstrates how to balance hydrogen, then nitrogen, and finally oxygen by doubling the compound to achieve balance. The summary underscores the step-by-step method for balancing equations, emphasizing the logical sequence and the importance of considering polyatomic ions as single units.
Takeaways
- 🔍 **Balancing Equations:** The process involves ensuring that the number of atoms for each element is the same on both sides of the equation.
- 📝 **Initial Tally:** Start by making a table to tally the number of atoms for each element present in the reaction.
- ✅ **Balance Calcium First:** In the first equation, balancing calcium by placing a coefficient of 3 in front of CaCl2 helps to balance the calcium atoms.
- 🔄 **Consider Polyatomic Ions:** Phosphate (PO4) is a polyatomic ion and should be balanced as a whole rather than individual atoms of phosphorus and oxygen.
- 🧂 **Address Sodium and Chlorine Together:** After balancing calcium and phosphate, adjusting the coefficient for NaCl helps to balance both sodium and chlorine atoms simultaneously.
- 🔢 **Use Coefficients Wisely:** Changing the coefficients in front of molecules can help balance multiple elements at once.
- 🔥 **Combustion Reaction Tip:** When balancing combustion reactions, it's often easier to balance elements that are alone in their molecule last, like oxygen in O2.
- 🤝 **Balance Carbon and Hydrogen Sequentially:** In the second equation, balance carbon and then hydrogen, adjusting the coefficients for CO2 and H2O respectively.
- 🌐 **Balance Oxygen Last:** If oxygen is initially balanced, it can be adjusted last to finalize the equation.
- 🛑 **Recheck After Changes:** After making changes to balance one element, recheck and rebalance other elements as necessary.
- 🤔 **Strategic Approach:** Start with elements that have the least complex chemistry to balance, and save elements in their elemental form (like H2) for last as they offer the most flexibility.
- ⚖️ **Final Check:** Once all elements are balanced, double-check the equation to ensure that the law of conservation of mass is upheld.
Q & A
What is the main topic discussed in the transcript?
-The main topic discussed in the transcript is the process of balancing chemical equations.
Why are chemical equations important to balance?
-Chemical equations are important to balance because they must accurately represent the conservation of mass, ensuring that the number of atoms for each element is the same on both sides of the equation.
What is the first step in balancing the given equations?
-The first step is to create a tally of the number of atoms of each element on both sides of the equation to identify which elements are not balanced.
How does one balance the equation involving CaCl2 and Na3PO4?
-The balance is achieved by adjusting the coefficients in front of the compounds to ensure that the number of atoms for each element is equal on both sides of the equation. For example, a coefficient of 3 is placed in front of CaCl2 and a coefficient of 6 is placed in front of NaCl.
What is a polyatomic ion?
-A polyatomic ion is a group of two or more atoms that are bonded together and function as a single ion with a specific charge, such as the phosphate ion (PO4^3-).
Why is it suggested to balance elements that are by themselves last?
-Elements that are by themselves, such as O2 or N2, are often balanced last because they provide the most flexibility. The coefficients for these elements can be adjusted without affecting the balance of other elements in the equation.
In the combustion reaction of C2H6O with O2, what is the final coefficient in front of O2?
-The final coefficient in front of O2 in the balanced equation is 3, to balance the seven oxygen atoms on the right side with the three on the left.
How many hydrogen atoms are there on the right side of the equation after balancing C8H18 with H2O?
-After balancing, there are 34 hydrogen atoms on the right side of the equation, which comes from 18 hydrogen atoms in C8H18 and 16 from eight water molecules (H2O).
What is the strategy for balancing the equation involving NH4NO3?
-The strategy involves first balancing the hydrogen atoms by adjusting the coefficient in front of H2O, then balancing the oxygen atoms by adding more water molecules, and finally ensuring nitrogen balance by adjusting the coefficient in front of N2.
Why is it necessary to re-evaluate the balance of elements after making changes to one side of the equation?
-Changes to one side of the equation can affect the balance of other elements due to the conservation of mass. It is necessary to re-evaluate and make further adjustments to ensure that all elements are balanced across the entire equation.
What is the final coefficient in front of H2 in the equation involving CO and H2 yielding C8H18 and H2O?
-The final coefficient in front of H2 is 17, which provides the necessary 34 hydrogen atoms on the left side to balance the equation.
Outlines
🔍 Balancing Equations with CaCl2 and Na3PO4
The paragraph introduces the task of balancing chemical equations. It provides a step-by-step guide on how to balance the equation involving CaCl2 and Na3PO4. The process involves creating a tally table for each element, comparing the number of atoms on both sides of the equation, and then applying coefficients to balance the atoms. The paragraph emphasizes the importance of balancing elements like calcium, chlorine, sodium, and the polyatomic phosphate ion as a whole, before addressing the individual atoms within it. The strategy concludes with the balanced equation for the reaction.
🔥 Balancing a Combustion Reaction
This paragraph discusses the balancing of a combustion reaction with C2H6O and O2 as reactants and CO2 and H2O as products. The summary explains the initial tally of carbon, hydrogen, and oxygen atoms on both sides of the equation. It then proceeds to balance the equation by adjusting coefficients for carbon and hydrogen first, as per the tip to balance elements that are alone last. The paragraph highlights the process of incrementing coefficients for CO2 and H2O to achieve balance for carbon and hydrogen, respectively. Finally, it addresses the balancing of oxygen by adjusting the coefficient for O2, resulting in a fully balanced chemical equation.
⚙️ Balancing Equations with CO, H2, and C8H18
The third paragraph focuses on balancing an equation with CO and H2 as reactants and C8H18 and H2O as products. The summary outlines the initial tally of carbon, hydrogen, and oxygen atoms. It then details the step-by-step process of balancing carbon first by adjusting the coefficient for CO, which subsequently affects the oxygen balance. The paragraph explains the need to balance oxygen next, followed by hydrogen, which is the last element to adjust due to its standalone nature in the molecule H2. The strategy concludes with the balanced equation for the reaction.
Mindmap
Keywords
💡Balancing Equations
💡Polyatomic Ions
💡Coefficient
💡Conservation of Mass
💡Combustion Reaction
💡Tally
💡Element
💡Reactants and Products
💡Stoichiometry
💡Law of Conservation of Charge
💡Combining Reaction
Highlights
Introduction to the process of balancing chemical equations, emphasizing the need for equal number of atoms on both sides of the equation.
Use of a table to tally the number of atoms of each element involved in the reaction to ensure balance.
Strategy of starting with the most complex element or polyatomic ion to balance the equation.
Explanation of how to balance the equation for the reaction between CaCl2 and Na3PO4 to form Ca3(PO4)2 and NaCl.
Technique of multiplying the entire polyatomic ion (phosphate ion) to balance phosphorus and oxygen simultaneously.
Adjustment of coefficients for sodium and chlorine to balance the equation, considering their presence in NaCl.
Combustion reaction example of C2H6O with O2 producing CO2 and H2O, demonstrating the balancing process.
Tip to balance elements that are by themselves (like O2, N2, H2) last for maximum flexibility.
Balancing carbon first in the combustion reaction by adjusting the coefficient for CO2.
Balancing hydrogen by adjusting the coefficient for water (H2O) to match the number of hydrogen atoms.
Final step of balancing oxygen by manipulating the coefficient for O2 to achieve equality on both sides.
Third example of balancing the equation for CO + H2 yielding C8H18 + H2O, emphasizing the strategy of balancing elements step by step.
Balancing carbon by adjusting the coefficient for CO to match the number of carbon atoms on both sides.
Addressing the balance of oxygen after adjusting for carbon, by adding a coefficient to the H2O molecule.
Final balance of hydrogen by adjusting the coefficient for H2, since it is alone on the reactant side.
Fourth example of balancing the decomposition reaction of NH4NO3 yielding N2, O2, and H2O.
Balancing hydrogen by adjusting the coefficient for water (H2O) to match the number of hydrogen atoms on both sides.
Balancing oxygen by adding additional water molecules to increase the oxygen count on the product side.
Final balance of nitrogen by adjusting the coefficient for N2 to match the number of nitrogen atoms on both sides.
Transcripts
Browse More Related Video

GCSE Chemistry - Balancing Chemical Equations #4

How To Balance Chemical Equations
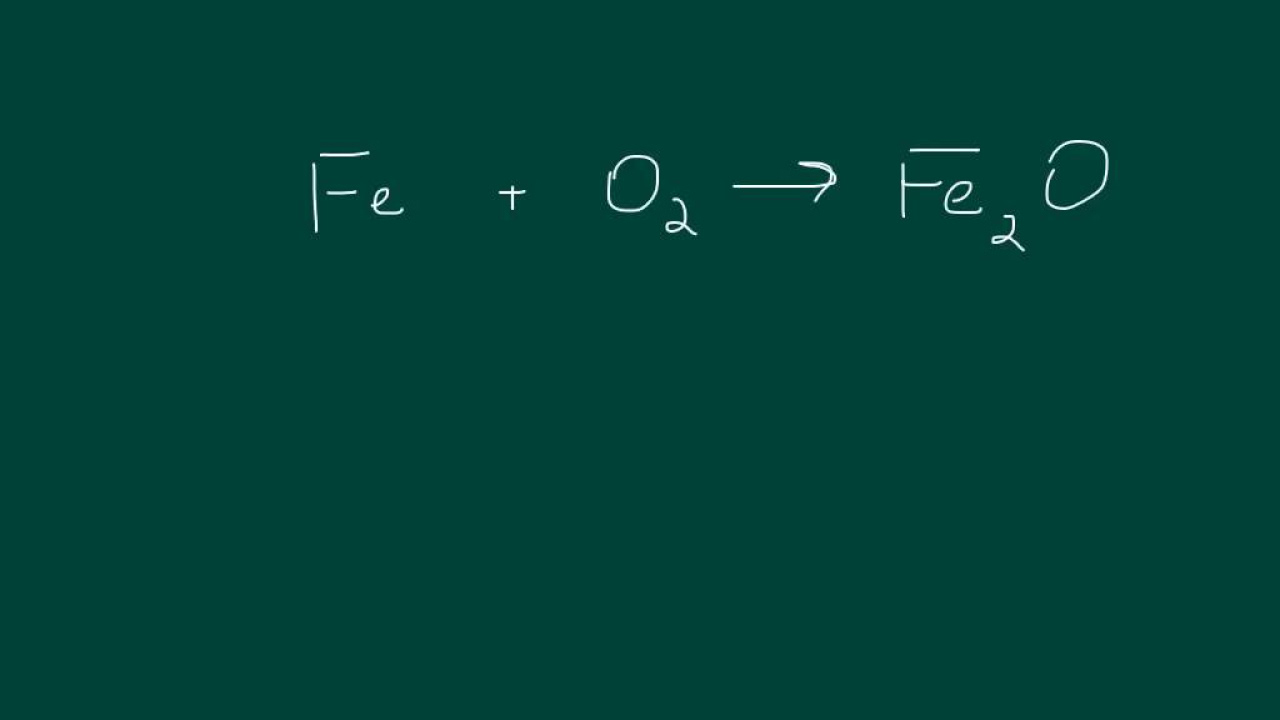
Synthesis Reactions

Balancing chemical equations | Chemical reactions | High school chemistry | Khan Academy

Balancing another combustion reaction | Chemical reactions | High school chemistry | Khan Academy

Balancing Chemical Equations Step by Step Practice Problems | How to Pass Chemistry
5.0 / 5 (0 votes)
Thanks for rating: