Unit 4.5 - Stoichiometry
TLDRThis chemistry lesson delves into the principles of stoichiometry, emphasizing its mathematical nature and the importance of using a calculator. The instructor reviews the conversion between moles, particles, liters, and grams, highlighting the significance of Avogadro's number and molar mass. The lesson covers the concept of limiting reactants, theoretical yield, and percent yield, providing step-by-step problem-solving methods. It also addresses the complexities of gas stoichiometry, including standard temperature and pressure conditions, and the use of the ideal gas law. The video is designed to refresh students' knowledge and prepare them for advanced chemistry challenges.
Takeaways
- 📚 Stoichiometry is the study of quantities in chemical reactions, focusing on the amounts of reactants consumed and products produced.
- 🔢 A calculator is essential for the mathematical calculations involved in stoichiometry problems.
- 🔄 The process involves converting between moles, particles, liters, and grams using specific conversion factors like Avogadro's number and molar mass.
- 🌐 A graphical organizer is used to visually represent the conversion between known and unknown quantities in a stoichiometry problem.
- ⚖️ Moles are the central unit for conversion, and the molar ratio from balanced chemical equations is key to finding unknown amounts.
- 🧪 Limiting reactant is the first reactant to be completely consumed in a reaction, limiting the amount of product that can be formed.
- 🔍 To find the limiting reactant, perform separate stoichiometry calculations for each reactant and choose the smaller resulting product amount.
- 📉 Excess reactant is the reactant that remains after the limiting reactant is consumed, and it does not affect the amount of product formed.
- 📊 Percent yield is calculated by dividing the actual yield by the theoretical yield, expressing the efficiency of a chemical reaction.
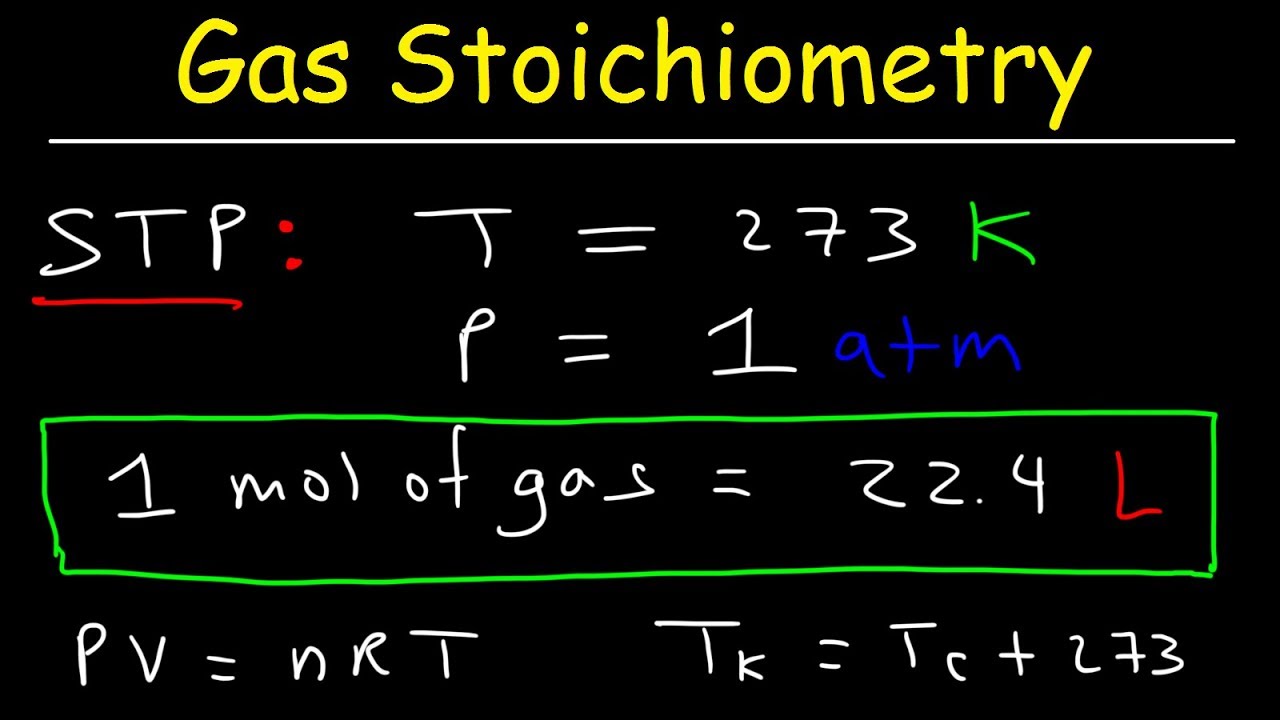
- 🌡️ For gases at Standard Temperature and Pressure (STP), 1 mole of any gas occupies 22.4 liters, a key conversion for gas stoichiometry.
- 🌌 The ideal gas law (PV=nRT) is used for calculating moles or volumes of gases when reactions do not occur at STP or when additional gas properties are known.
Q & A
What is stoichiometry and why is it important in chemistry?
-Stoichiometry is the study of the quantities of reactants consumed and products produced in a chemical reaction. It is important because it allows chemists to determine unknown amounts in a reaction using known quantities, which is essential for understanding and predicting chemical processes.
What are the conversion factors used when going back and forth between moles and grams of a substance?
-The conversion factor used when going back and forth between moles and grams of a substance is the molar mass, which can be found on the periodic table.
What is Avogadro's number and how is it used in stoichiometry problems?
-Avogadro's number is the number of particles (atoms, molecules, ions, etc.) in one mole of a substance, approximately 6.022 x 10^23. It is used as a conversion factor when converting between moles and the number of particles.
What is the significance of the number 22.4 liters/mole in stoichiometry?
-The number 22.4 liters/mole is significant because it represents the volume occupied by one mole of an ideal gas at standard temperature and pressure (STP). It is used to convert between moles and liters for gases.
Why is it necessary to use a balanced chemical equation for stoichiometry calculations?
-A balanced chemical equation is necessary for stoichiometry calculations because it accurately represents the molar ratios of reactants and products in a chemical reaction. Using an unbalanced equation would lead to incorrect stoichiometric calculations.
What is a limiting reactant and how does it affect the amount of product formed in a reaction?
-A limiting reactant is the reactant that is completely consumed first in a chemical reaction, thus limiting the amount of product that can be formed. The other reactant(s) present in excess do not limit the reaction.
How can you determine which reactant is the limiting reactant in a reaction?
-To determine the limiting reactant, you perform separate stoichiometry calculations using the given amounts of each reactant to see which one would produce a smaller amount of product. The reactant that results in the smaller amount of product is the limiting reactant.
What is the theoretical yield in a chemical reaction and how does it differ from the actual yield?
-The theoretical yield is the maximum amount of product that can be produced in a reaction assuming 100% efficiency with no errors. The actual yield is the amount of product actually obtained from the reaction, which is usually less than the theoretical yield due to real-world inefficiencies and errors.
What is percent yield and how is it calculated?
-Percent yield is a measure of the efficiency of a chemical reaction, calculated as the ratio of the actual yield to the theoretical yield, expressed as a percentage. It is calculated using the formula: (actual yield / theoretical yield) * 100%.
Why is it important to consider the conditions of standard temperature and pressure (STP) when dealing with gases in stoichiometry problems?
-It is important to consider STP conditions when dealing with gases because the volume of a gas can change significantly with temperature and pressure. At STP, the volume of one mole of an ideal gas is fixed at 22.4 liters, which is used for stoichiometry calculations involving gases.
What is the ideal gas law and when is it used in stoichiometry problems involving gases?
-The ideal gas law is the equation PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature in Kelvin. It is used in stoichiometry problems when the reaction involving gases is not at STP or when the volume or the number of moles of a gas needs to be calculated based on other given conditions.
What is the significance of partial pressures in the context of gases collected over water?
-Partial pressures are significant when a gas is collected over water because the total pressure in the collection space is the sum of the partial pressures of the gas and water vapor. To find the pressure of the gas alone, you must subtract the vapor pressure of water from the total pressure.
Outlines
📚 Stoichiometry Basics and Problem Solving
This paragraph introduces the topic of stoichiometry, emphasizing its importance in understanding the quantities of reactants and products in chemical reactions. The instructor prepares students for a review and new material involving mathematical calculations and problem-solving. The summary includes the use of a graphic organizer to convert between moles, particles, liters, and grams. It also explains the necessity of using Avogadro's number, molar mass, and the 22.4 L/mole volume for gases at STP (Standard Temperature and Pressure) for these conversions. The paragraph concludes with an introduction to a simple stoichiometry problem involving the calculation of grams of iron(III) oxide needed for a reaction.
🔍 Delving into Stoichiometry with Molarity and Limiting Reactants
The second paragraph continues the discussion on stoichiometry, focusing on the concept of molarity and how to convert it into moles for use in stoichiometric calculations. The instructor guides through a step-by-step problem involving hydrochloric acid, emphasizing the importance of balancing chemical equations for accurate calculations. The paragraph also revisits the concept of limiting reactants, explaining how to identify and calculate them in a chemical reaction. The summary includes the method for determining the limiting reactant and the excess reactant, and how to calculate the amount of product formed based on the limiting reactant.
🔄 Understanding Limiting Reactants and Excess Reactants in Stoichiometry
This paragraph delves deeper into the concept of limiting and excess reactants, providing a step-by-step method to solve stoichiometry problems involving multiple reactants. The instructor explains how to perform separate calculations for each reactant to determine the limiting reactant, which dictates the maximum amount of product that can be formed. The summary includes an example problem involving the production of ammonia from nitrogen and hydrogen, illustrating the process of identifying the limiting reactant and calculating the theoretical yield of product and the amount of excess reactant remaining.
📉 Visualizing Chemical Reactions and Calculating Yields
The fourth paragraph introduces visual problems in stoichiometry, where students must interpret diagrams representing chemical reactions and determine the correct outcome based on the reactants provided. The instructor discusses the concept of theoretical yield versus actual yield and introduces the calculation of percent yield, which measures the efficiency of a chemical reaction. The summary includes a step-by-step guide to solving a multiple-choice problem involving the reaction of hydrogen and nitrogen, emphasizing the importance of identifying the limiting reactant and calculating the correct amount of product formed.
🌡️ Advanced Stoichiometry with Gases at Non-STP Conditions
This paragraph addresses more complex stoichiometry problems involving gases that are not at standard temperature and pressure. The instructor explains the use of the ideal gas law (PV=nRT) to calculate moles or volumes of gases when reactions do not occur at STP. The summary includes a detailed explanation of how to approach problems where the reaction involves gases at non-STP conditions, and how to use stoichiometry in conjunction with the ideal gas law to find the required quantities of reactants or products.
📊 Calculating Theoretical and Percent Yield in Stoichiometry
The sixth paragraph focuses on the calculation of theoretical yield and percent yield in stoichiometry problems. The instructor provides a step-by-step guide on how to use stoichiometry to calculate the theoretical yield of a product and then determine the percent yield based on the actual yield obtained from an experiment. The summary includes an example problem involving the reaction of aluminum with bromine to produce aluminum bromide, illustrating the process of calculating theoretical and percent yield.
💧 Special Considerations for Gases Collected Over Water
The final paragraph discusses special considerations for stoichiometry problems involving gases collected over water, where the collected gas may contain both the product gas and water vapor. The instructor explains the concept of partial pressures and how to account for water vapor pressure when calculating the volume or moles of the product gas. The summary includes a problem involving the decomposition of potassium chlorate and the calculation of the volume of oxygen gas produced, taking into account the vapor pressure of water at the given temperature.
Mindmap
Keywords
💡Stoichiometry
💡Moles
💡Molar Mass
💡Avogadro's Number
💡Balanced Equation
💡Molar Ratio
💡Standard Temperature and Pressure (STP)
💡Limiting Reactant
💡Theoretical Yield
💡Percent Yield
💡Ideal Gas Law
Highlights
Stoichiometry involves the study of quantities in chemical reactions, using known amounts to determine unknowns.
A graphical organizer is used to convert between moles, particles, liters, and grams.
Avogadro's number and molar mass are key conversion factors in stoichiometry.
The molar ratio from a balanced chemical equation is essential for stoichiometric calculations.
The concept of limiting reactant determines the maximum amount of product that can be formed.
Two separate stoichiometry problems are often needed to identify the limiting reactant.
Theoretical yield represents the maximum product amount in an ideal reaction.
Actual yield is the amount of product obtained in a real-world experiment.
Percent yield measures the efficiency of a reaction, calculated as actual yield over theoretical yield.
Gas stoichiometry at Standard Temperature and Pressure (STP) uses the 22.4 L/mole conversion factor.
The ideal gas law (PV=nRT) is used for non-STP conditions to calculate moles or volume of a gas.
When gases are collected over water, the vapor pressure of water must be accounted for in the calculations.
In stoichiometry, understanding the context of the problem is crucial for selecting the correct method or formula.
Examples and practice problems are used throughout the lesson to illustrate stoichiometry concepts.
The lesson concludes with a comprehensive problem involving the ideal gas law and stoichiometry.
The importance of distinguishing between different gases and conditions in stoichiometric calculations is emphasized.
The lesson aims to clarify and reinforce understanding of stoichiometry, with a focus on practical application.
Transcripts
Browse More Related Video

Gas Stoichiometry: Equations Part 1
Gas Stoichiometry Problems
Stoichiometry Tutorial: Step by Step Video + review problems explained | Crash Chemistry Academy

Stoichiometry: Limiting Reactant, Left Over Excess Reactant, Percent Yield | Study Chemistry With Us
Know This For Your Chemistry Final Exam - Stoichiometry Review
9.2 Gas Laws including the Ideal Gas Law | High School Chemistry
5.0 / 5 (0 votes)
Thanks for rating: