Stoichiometry Tutorial: Step by Step Video + review problems explained | Crash Chemistry Academy
TLDRThis tutorial on stoichiometry explains how to use chemical equations to determine the relationships between reactants and products in a chemical reaction. It emphasizes the importance of coefficients, which represent ratios and can be used to calculate the amounts of substances involved in a reaction. The video covers how to convert between different units, such as moles, mass, and particles, using molar ratios and Avogadro's number. It demonstrates the process of mole-mole conversion, mass-to-mass stoichiometry, and how to handle problems involving gas volumes at standard temperature and pressure. The tutorial also includes practice problems to illustrate the application of stoichiometry in determining the quantities of reactants needed or products formed in a reaction, showcasing its utility in chemical calculations.
Takeaways
- 🔍 **Stoichiometry Definition**: Stoichiometry is the calculation of relative quantities of reactants and products in chemical reactions based on the balanced chemical equation.
- ⚖️ **Coefficients as Ratios**: Coefficients in a balanced chemical equation represent the ratio of reactants to products, which remains constant regardless of the scale of the reaction.
- 🧪 **Understanding Coefficients**: Coefficients can indicate specific amounts of particles or the ratio of reactants and products, which is crucial for stoichiometric calculations.
- 🌐 **Mass to Mass Calculations**: Stoichiometry allows chemists to determine the amount of product formed or the amount of one reactant needed for a given quantity of another reactant.
- 📐 **Molar Ratios**: Coefficients are molar ratios and can represent any amount, including molar amounts, which are especially useful when dealing with large numbers of particles.
- 🔄 **Conservation of Mass**: The mole-mole conversion is central to stoichiometry, allowing for the conversion from moles of one substance to moles of another based on the balanced equation.
- 📊 **Unit Conversions**: Stoichiometry often involves multiple unit conversions, such as from mass to moles, using molar masses, and then to the desired product or reactant.
- 🔢 **Dimensional Analysis**: Stoichiometric calculations are structured similarly to dimensional analysis, ensuring that units cancel out to leave the desired unit (e.g., grams of product).
- 🔬 **Avogadro's Number**: Avogadro's number (6.02 x 10²³) is used to convert between moles and the number of particles, such as atoms, ions, molecules, or formula units.
- 🌡️ **Gas Volume at STP**: At standard temperature and pressure, a mole of gas particles occupies 22.4 liters, which is useful for converting between gas volume and moles in stoichiometric calculations.
- 🔠 **Balanced Equations Essential**: Balanced chemical equations are necessary for performing stoichiometry, as they provide the correct mole-mole relationships between reactants and products.
Q & A
What is stoichiometry?
-Stoichiometry is the calculation of quantitative relationships between reactants and products in a chemical reaction based on the reaction's balanced chemical equation.
What are coefficients in a chemical equation?
-Coefficients in a chemical equation represent the ratio of reactants to products in a balanced chemical equation. They can also indicate the specific number of particles of a substance involved in the reaction.
How do you balance a chemical equation?
-A chemical equation is balanced by adjusting the coefficients (the numbers placed in front of the chemical formulas) to ensure that the number of atoms of each element on the reactant side equals the number on the product side.
What is the relationship between moles and the coefficients in a chemical equation?
-The coefficients in a chemical equation represent the molar ratios between reactants and products. For instance, a coefficient of '3' for hydrogen molecules indicates that three moles of hydrogen are involved in the reaction.
How is stoichiometry used to determine the amount of product formed in a reaction?
-Stoichiometry is used to determine the amount of product formed by using the balanced chemical equation's coefficients to calculate the molar ratios between reactants and products, and then converting these ratios to actual amounts using molar masses.
What is the role of Avogadro's number in stoichiometric calculations?
-Avogadro's number (6.02 x 10^23) is used to convert between the number of particles (atoms, molecules, ions, etc.) and moles. Since one mole of any substance contains Avogadro's number of particles, this constant is crucial for stoichiometric calculations involving particles.
How do you convert mass to moles in stoichiometry?
-To convert mass to moles, you use the substance's molar mass, which is the mass of one mole of that substance. You divide the mass of the substance by its molar mass to get the number of moles.
What is the concept of mole-mole conversion in stoichiometry?
-Mole-mole conversion is a central part of stoichiometric calculations where you use the balanced chemical equation's coefficients to find the relationship between the moles of different substances in a reaction. This allows you to calculate the amount of one substance needed to react with or produce a certain amount of another substance.
How does the mole concept help in handling large amounts of particles in chemical reactions?
-The mole concept simplifies the handling of large amounts of particles by allowing chemists to work with molar ratios, which remain constant regardless of the actual number of particles involved. This makes it possible to predict chemical reaction outcomes on both a small and an industrial scale.
What is the importance of maintaining consistent units in stoichiometric calculations?
-Maintaining consistent units is crucial for accuracy in stoichiometric calculations. It ensures that the conversion factors between different quantities (like mass to moles or moles to volume) are correctly applied, leading to correct results. If units do not cancel out as expected, it indicates a mistake in the setup of the calculation.
How can stoichiometry be extended beyond mass-to-mass calculations?
-Stoichiometry can be extended to include calculations involving particles, volumes of gases at standard temperature and pressure (STP), and other quantities that can be converted to moles. This versatility allows chemists to predict outcomes and carry out calculations for a wide range of chemical scenarios.
Outlines
🔍 Introduction to Stoichiometry
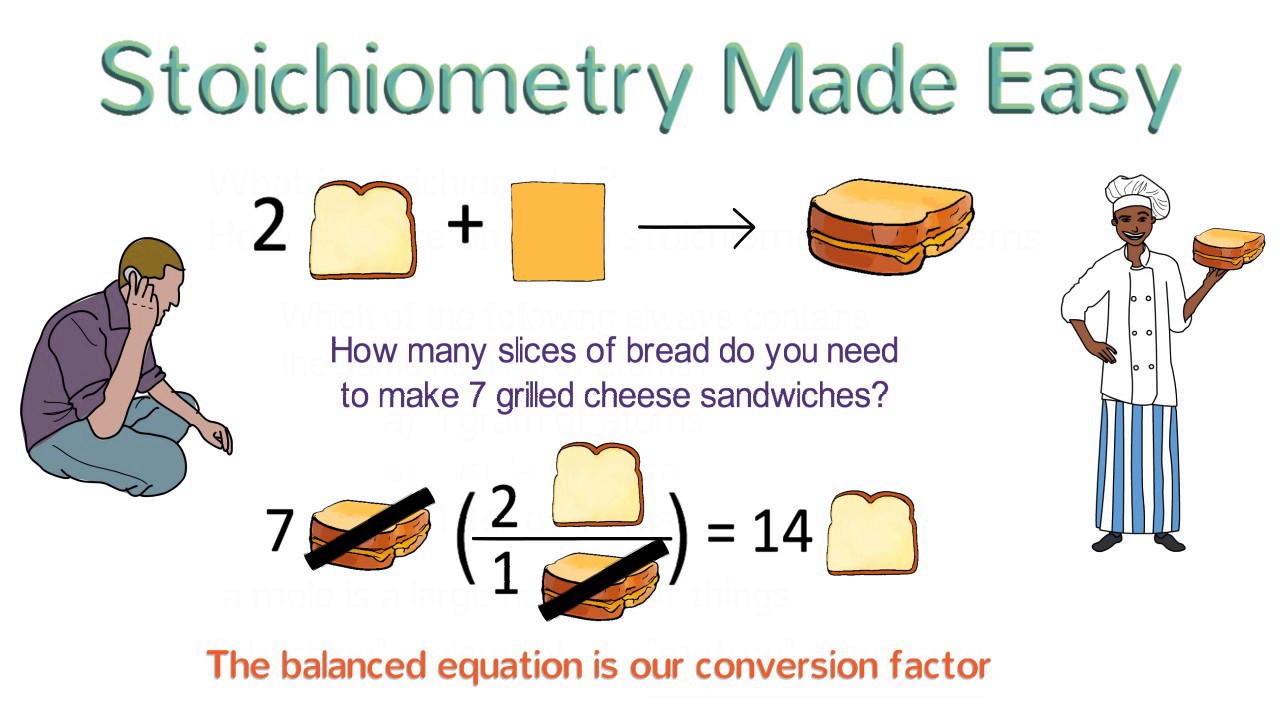
This paragraph introduces the concept of stoichiometry as the mathematical relationship between reactants and products in a balanced chemical equation. It emphasizes the importance of understanding coefficients, which can represent specific amounts of particles or ratios of reactants and products. The example of the reaction between hydrogen (H₂) and nitrogen (N₂) to form ammonia (NH₃) is used to illustrate how stoichiometry works, highlighting the concept that the reaction always occurs in a three-to-one-to-two ratio, regardless of the number of molecules involved. The paragraph also explains how these ratios translate into molar ratios, allowing chemists to predict the quantities involved in chemical reactions.
📐 Stoichiometric Calculations and Mole-Mole Conversions
This section delves into the practical application of stoichiometry by demonstrating how to use coefficient ratios to calculate the amounts of reactants and products in a chemical reaction. It explains the process of mole-mole conversion, where given a certain amount of one substance, you can determine the required or produced amount of another substance using the balanced chemical equation's coefficients. The paragraph also addresses the challenge of determining the number of moles directly, and how mass measurements can be used in conjunction with molar masses to facilitate these calculations. The concept of dimensional analysis is introduced, showing how to set up and solve stoichiometric problems involving multiple unit conversions, using the example of producing ammonia from nitrogen.
🔬 Advanced Stoichiometry: Beyond Mass-to-Mass Calculations
The final paragraph extends the application of stoichiometry to include calculations involving different quantities, such as particles or gas volumes. It outlines how to convert between mass, moles, and particles using Avogadro's number and how to work with gas volumes at standard temperature and pressure. The paragraph provides a comprehensive map for various types of stoichiometric calculations, emphasizing the central role of mole-mole calculations. It also presents additional practice problems, such as calculating the mass of oxygen needed for a reaction with ammonia and the mass of carbon dioxide produced from the combustion of ethane. The summary concludes with an example of finding the mass of water produced from a given number of ethane molecules, reinforcing the foundational principles of stoichiometry.
Mindmap
Keywords
💡Stoichiometry
💡Coefficients
💡Molar Ratios
💡Mole-Mole Conversion
💡Molar Mass
💡Dimensional Analysis
💡Avogadro's Number
💡Standard Temperature and Pressure (STP)
💡Chemical Equation
💡Combustion Reaction
💡Balanced Equation
Highlights
Stoichiometry is defined as the mathematical relationship between reactants and products in a chemical equation
Coefficients in a chemical equation represent ratios of reactants to products
The ratio of reactants to products remains constant regardless of the amount of reactants used
Coefficients can represent specific amounts of particles or molar ratios
Mole-mole conversion is the central part of stoichiometric calculations
Molar mass can be used to convert between mass and moles of a substance
Stoichiometry can be used to determine the amount of product formed or reactant needed in a reaction
The mole-mole conversion ratio is used to relate the amounts of different substances in a reaction
Avogadro's number can be used to convert between moles and particles in a reaction
At standard temperature and pressure, 1 mole of gas occupies 22.4 liters
A map or pathway can guide conversions between mass, moles, particles, and gas volume in stoichiometry problems
The balanced chemical equation provides the necessary coefficients for stoichiometric calculations
The mole-mole conversion ratio is key for converting between different substances in a reaction
Dimensional analysis and unit cancellation are crucial for accurate stoichiometric calculations
Stoichiometry allows chemists to determine the amount of product formed or reactant needed in a reaction
Practice problems help build proficiency in stoichiometric calculations and conversions
Stoichiometry has practical applications in chemistry for determining reactant and product amounts
Transcripts
Browse More Related Video

Step by Step Stoichiometry Practice Problems | How to Pass Chemistry
Stoichiometry | Mole to mole | Grams to grams | Mole to grams | Grams to mole | Mole ratio
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6
Gas Stoichiometry Problems
Solution Stoichiometry - Finding Molarity, Mass & Volume
Stoichiometry Made Easy: Stoichiometry Tutorial Part 1
5.0 / 5 (0 votes)
Thanks for rating: