Hybridization, Orbital Overlap, and Bond Length
TLDRThis educational script discusses the concept of bond length in relation to hybridization. It explains how the s-character in hybrid orbitals affects bond length, with more s-character leading to shorter and stronger bonds. The script uses carbon atoms as an example, comparing single, double, and triple bonds, and how sp3, sp2, and sp hybridization influence bond length. It concludes by ranking five different bonds from longest to shortest based on their hybridization, with sp3 bonds being the longest and sp-sp2 bonds the shortest.
Takeaways
- 🔬 The script discusses the ranking of single bonds in order of decreasing bond length based on hybridization and s-character.
- 📚 Ethane and acetylene are used as examples to illustrate the differences between single and triple bonds and their respective hybridizations.
- 🌐 Hybridization plays a key role in determining bond length, with sp3, sp2, and sp hybrid orbitals being compared.
- 📊 The percentage of s-character in hybrid orbitals is crucial: sp3 has 25% s-character, sp2 has 33.33%, and sp has 50%.
- ⚠️ Comparing bond lengths should be done within the same row of the periodic table to account for energy level differences.
- 🔑 The more s-character in a bond, the shorter and stronger the bond will be due to the S orbital's proximity to the nucleus.
- 🔍 The script explains that the triple bond in acetylene is shorter than the single bond in ethane because of higher s-character.
- 📝 The ranking of the bonds is based on the hybridization of the atoms involved: sp3, sp2, and sp.
- 📉 Bond number five, being sp3-sp3, is identified as the longest single bond due to the least s-character.
- 📈 Bond number one, formed from sp3 and sp2 hybrid orbitals, is the second longest, followed by bond number four (sp-sp3).
- 📊 Bond number two, involving an sp hybrid orbital, is the shortest due to the highest s-character, making it the strongest but with the shortest length.
Q & A
What is the main topic discussed in the video script?
-The main topic discussed in the video script is the comparison of bond lengths of single bonds in different hybridization states, specifically focusing on carbon atoms.
Why are triple bonds generally shorter than single bonds?
-Triple bonds are generally shorter than single bonds because they have more s character in their hybrid orbitals, which results in a shorter bond length due to the s orbital being closer to the nucleus than the p orbital.
What is the s character percentage in an sp3 hybrid orbital?
-In an sp3 hybrid orbital, there is 25% s character, as it is a hybrid of one s orbital and three p orbitals.
How does the s character in hybrid orbitals affect bond strength and length?
-The more s character in a hybrid orbital, the stronger and shorter the bond will be, because the s orbital is closer to the nucleus, leading to a stronger electrostatic attraction between the nuclei and the bonding electrons.
What is the hybridization of carbon atoms in ethane and acetylene?
-In ethane, carbon atoms are sp3 hybridized, while in acetylene, the carbon atoms involved in the triple bond are sp hybridized.
What is the role of s and p orbitals in determining bond length?
-The s orbital, being closer to the nucleus, contributes to a shorter bond length when it has a higher s character in the hybrid orbital. The p orbital contributes to a longer bond length when the hybrid orbital has less s character.
How does the video script rank the single bonds in order of decreasing bond length?
-The script ranks the single bonds based on the hybridization of the atoms involved, with sp3 hybridization resulting in the longest bond and sp hybridization resulting in the shortest bond.
What is the hybridization of the carbon atoms in bond number one?
-Bond number one is formed from the overlap of an sp3 hybrid orbital and an sp2 hybrid orbital.
What is the hybridization of the carbon atoms in bond number two?
-Bond number two is formed from the overlap of an sp2 hybrid orbital and an sp hybrid orbital.
How does the script determine the longest and shortest single bonds among the given options?
-The script determines the longest and shortest single bonds by comparing the s character in the hybrid orbitals of the atoms involved in each bond, with more s character resulting in a shorter bond.
What is the final ranking of the single bonds from longest to shortest according to the script?
-The final ranking is bond number five (sp3-sp3) being the longest, followed by bond number one (sp3-sp2), then bond number four (sp-sp3), bond number two (sp2-sp), and bond number three (sp-sp) being the shortest.
Outlines
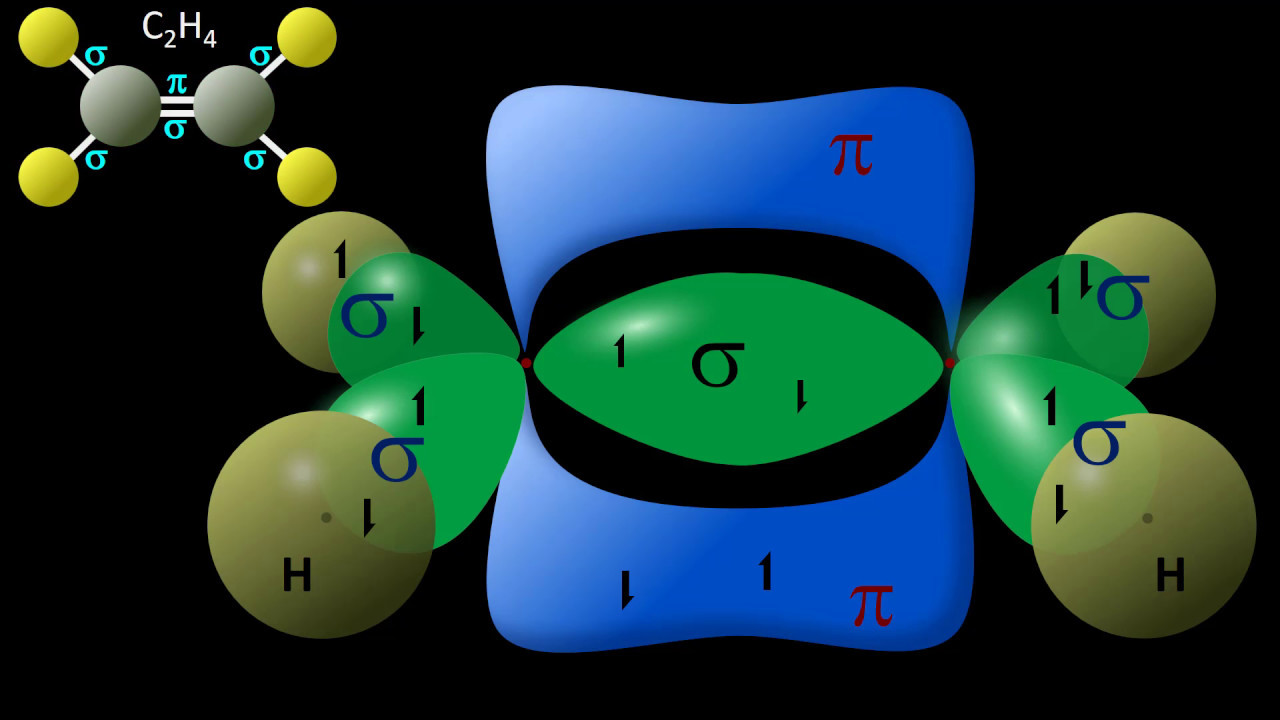
🔬 Understanding Bond Lengths and Hybridization
This paragraph introduces the concept of comparing bond lengths of single bonds by considering the hybridization of the atoms involved. It uses ethane and acetylene as examples to explain how the type of hybrid orbitals (sp3 in ethane and sp in acetylene) affects bond length, with triple bonds being shorter than single bonds due to increased s-character. The importance of comparing atoms in the same row of the periodic table is highlighted, and the role of s-character in determining bond strength and length is discussed. The paragraph also explains that the s orbital's proximity to the nucleus contributes to increased bond strength and decreased bond length with higher s-character.
📊 Ranking Single Bonds by Length Based on Hybridization
The second paragraph delves into the specifics of ranking five different single bonds by their lengths, starting with the longest. It explains the process of determining bond length by comparing the hybridization of the atoms involved, from sp3 to sp. The paragraph outlines that bonds with more s-character are shorter and stronger. It then provides a ranking of the bonds based on their hybridization: bond number five, being all sp3, is the longest; bond number one, an overlap of sp3 and sp2, is the second longest; bond number four, sp-sp3, is the third longest; bond number two, an overlap of sp2 and sp, is shorter than number four but longer than number three; and bond number three, sp-sp, is the shortest due to the highest s-character. The paragraph concludes by inviting viewers interested in further practice to check the description for additional resources.
Mindmap
Keywords
💡Bond length
💡Single bond
💡Hybridization
💡sp3 hybridization
💡sp2 hybridization
💡sp hybridization
💡s-character
💡Pi bond
💡Sigma bond
💡Acetylene
💡Ethane
Highlights
Ranking of single bonds in order of decreasing bond length.
Comparison of single bonds based on hybridization and s-character.
Explanation of why triple bonds are shorter than single bonds due to more s-character.
Introduction of hybridization concepts: sp3, sp2, and sp orbitals.
Calculation of s-character percentage in sp3 and sp hybrid orbitals.
Importance of s-character in bond length and strength.
Comparison of bond lengths based on s-character, with sp3 being the longest.
Hybridization of carbon atoms in ethane and acetylene.
Explanation of how s-orbital's proximity to the nucleus affects bond length.
Guidance on comparing elements in the same row of the periodic table for s-character.
Identification of the longest bond as the one with the least s-character (sp3-sp3).
Ranking of bond lengths based on hybridization: sp3-sp2, sp2-sp, sp-sp3, and sp3-sp3.
Determination of the shortest bond as the one with the most s-character (sp-sp).
Practical application of hybridization theory in predicting bond lengths.
Final ranking of the bonds from longest to shortest based on hybridization.
Invitation to view additional practice problems in the video description.
Transcripts
Browse More Related Video

1.3 Valence Bond Theory and Hybridization | Organic Chemistry
Hybrid Orbitals explained - Valence Bond Theory | Orbital Hybridization sp3 sp2 sp

Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory

Bond Strength and Bond Length

Potential Energy vs Internuclear Distance

Valence Bond Theory & Hybrid Atomic Orbitals
5.0 / 5 (0 votes)
Thanks for rating: