Ionization energy trends | Periodic table | Chemistry | Khan Academy
TLDRThis educational script delves into the concept of ions, explaining that they are atoms or molecules with an unequal number of protons and electrons, resulting in a net charge. It distinguishes between cations, with more protons than electrons, and anions, with the opposite. The script also introduces ionization energy, the energy required to remove an electron, and discusses how this energy varies across the periodic table, being low for alkali metals and high for noble gases, with trends influenced by an element's position within its group and period.
Takeaways
- 🔬 An ion is an atom or molecule that has a net charge due to an unequal number of protons and electrons.
- ⚛️ Neutrons are neutral and do not contribute to the charge of an atom or molecule.
- 🔋 Positive ions, or cations, are formed when an atom has more protons than electrons, while negative ions, or anions, have more electrons than protons.
- 🌐 The ionization energy is the energy required to remove an electron from an atom, which is a measure of how difficult it is to form a cation.
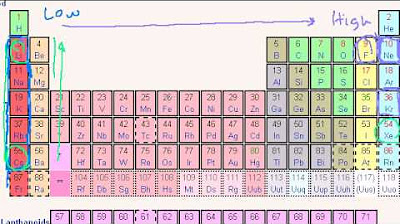
- 📊 Elements in the periodic table show trends in ionization energy; alkali metals have low ionization energy, making them eager to lose an electron.
- 💡 When an alkali metal loses an electron, it achieves a stable electron configuration similar to that of a noble gas.
- 🚫 Noble gases are very stable and have high ionization energy, making it difficult to remove an electron from them.
- ➡️ As you move from left to right across the periodic table, ionization energy generally increases, indicating it becomes harder to remove an electron.
- 🔽 Moving down a group in the periodic table, ionization energy decreases because outer electrons are further from the nucleus and less tightly bound.
- 📉 The ionization energy chart shows periodic trends that inspired the organization of the periodic table, with dips indicating exceptions to the general trend.
- 🌌 The ionization energy of larger atoms, like Radon, can be lower than that of smaller atoms like Hydrogen, due to the distance of outer electrons from the nucleus.
Q & A
What is an ion?
-An ion is an atom or molecule that has a net charge due to an unequal number of protons and electrons.
What causes an atom to become a positive ion or cation?
-An atom becomes a positive ion or cation when it has more protons than electrons.
How does an atom become a negative ion or anion?
-An atom becomes a negative ion or anion when it gains extra electrons, resulting in more electrons than protons.
What is the relationship between the number of protons and electrons in a neutral hydrogen atom?
-In a neutral hydrogen atom, the number of protons equals the number of electrons, which is one of each.
Why do alkali metals easily form cations?
-Alkali metals easily form cations because losing an electron allows them to achieve the stable electron configuration of the previous noble gas.
What is ionization energy?
-Ionization energy is the energy required to remove an electron from an atom or molecule, effectively making it a cation.
How does the ionization energy of elements vary across the periodic table?
-Ionization energy generally increases from left to right across the periodic table, and decreases from top to bottom within a group.
Why do noble gases have high ionization energies?
-Noble gases have high ionization energies because their electron configurations are already stable, making it difficult to remove an electron.
What is the significance of the term 'Octet Rule' in the context of ionization energy?
-The Octet Rule refers to the preference of atoms to have eight electrons in their outermost shell, which is a stable configuration. Elements near this configuration have higher ionization energies because they resist losing electrons that would disrupt this stability.
How does the distance of an electron from the nucleus affect ionization energy?
-The further an electron is from the nucleus, the weaker the electrostatic attraction and the easier it is to remove, resulting in lower ionization energy.
What causes the dips in ionization energy observed in the chart?
-The dips in ionization energy on the chart correspond to the noble gases, which have stable electron configurations and thus higher ionization energies compared to adjacent elements.
Why does the ionization energy of Radon, a noble gas, differ from that of Hydrogen?
-Although Radon is a noble gas with a stable electron configuration, its outermost electrons are further from the nucleus due to its larger atomic size, resulting in a lower ionization energy compared to Hydrogen.
Outlines
🔬 Understanding Ions and Ionization Energy
The video script begins with an exploration of the concept of ions, which are atoms or molecules with an unequal number of protons and electrons, resulting in a net charge. Positive ions, or cations, have more protons than electrons, while negative ions, or anions, have more electrons. The script uses hydrogen as an example of a positive ion and fluorine for a negative ion. It then introduces ionization energy, which is the energy required to remove an electron from an atom, and explains how this concept relates to the ease of turning elements into cations. The periodic table trends are discussed, highlighting that elements in group one, such as alkali metals, have a low ionization energy because they readily lose an electron to achieve a stable electron configuration similar to noble gases.
📊 Trends in Ionization Energy Across the Periodic Table
This paragraph delves into the trends of ionization energy as one moves across and down the periodic table. It explains that ionization energy generally increases from left to right and from bottom to top, indicating that it becomes progressively harder to remove an electron from an atom. Alkali metals, positioned on the left side, have low ionization energies because they easily lose an electron to achieve a noble gas configuration. Conversely, noble gases on the right side have high ionization energies due to their stable electron configurations. The paragraph also discusses the impact of additional electron shells on ionization energy, noting that electrons in outer shells are less tightly bound and thus easier to remove, as exemplified by cesium compared to lithium. The video script concludes by mentioning that even large atoms like radon, despite being noble gases, can have lower ionization energies than smaller atoms like hydrogen, due to the increased distance of their outermost electrons from the nucleus.
Mindmap
Keywords
💡Ion
💡Neutrons
💡Protons
💡Electrons
💡Cations
💡Anions
💡Ionization Energy
💡Alkali Metals
💡Noble Gases
💡Electron Configuration
💡Periodic Trends
Highlights
Ions are atoms or molecules with a net charge due to an unequal number of protons and electrons.
Neutrons are neutral and do not contribute to the charge of an atom or molecule.
Positive ions, or cations, have more protons than electrons.
Negative ions, or anions, have more electrons than protons.
An example of ion formation: Hydrogen becomes positively charged when it loses an electron.
Fluorine becomes a negatively charged ion by gaining an electron.
Ionization energy is the energy required to remove an electron from an atom.
Alkali metals have low ionization energy because they readily lose an electron to achieve a noble gas electron configuration.
Noble gases have high ionization energy due to their stable electron configurations.
Trend across the periodic table: Ionization energy increases from left to right.
Trend down the periodic table: Ionization energy increases from bottom to top.
The further an electron is from the nucleus, the easier it is to remove, resulting in lower ionization energy.
Ionization energy trends show a decrease as atomic size increases, even for noble gases.
Radon, despite being a noble gas, has a lower ionization energy than hydrogen due to the distance of its outer electrons from the nucleus.
The periodic table's trends were discovered by observing patterns in ionization energies.
Dips in ionization energy can be observed and theorized within the periodic table's trends.
The addition of D block elements in the periodic table affects the observed trends in ionization energy.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: