The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton
TLDRThe video script explores the ubiquitous nature of acids and bases in our daily lives, from food to cleaning products and even our own bodies. It delves into the historical definitions of acids and bases based on their properties, contrasting with modern molecular understanding. The script explains the interaction of molecules in water through the exchange of protons and electrons, highlighting the aggressiveness of strong acids and bases versus the more reserved behavior of weak ones. It emphasizes water's role as a mediator in acid-base chemistry, facilitating neutralization. The engaging narrative concludes by praising water as a steadfast participant in chemical reactions without seeking gain, a testament to its essential and unique position in the chemical economy.
Takeaways
- 🌟 Acids and bases are ubiquitous in our daily lives, being key components in various products like food, soap, and fertilizers.
- 🧪 The human body exhibits natural acidity and alkalinity, with stomachs being acidic and blood being slightly basic.
- 🍲 Ancient definitions of acids and bases were based on their observable properties, such as sour taste and metal corrosion for acids, and slippery feel with proton-accepting ability for bases.
- 💧 When molecules dissolve in water, they interact by exchanging protons (hydrogen ions) and electrons, which is the basis of their acidic or basic behavior.
- ⚡️ Protons carry a positive charge, and electrons carry a negative charge, influencing how molecules donate or accept these particles.
- 💣 Strong acids readily donate protons to their surroundings, while strong bases are eager to accept protons, often taking them from water molecules.
- 🔄 Weak acids and bases partially donate or accept protons in water, reaching an equilibrium where only a small fraction of their molecules react.
- 🥗 Common household substances like vinegar (a weak acid) and ammonia (a weak base) demonstrate that even weak acids and bases can have noticeable effects.
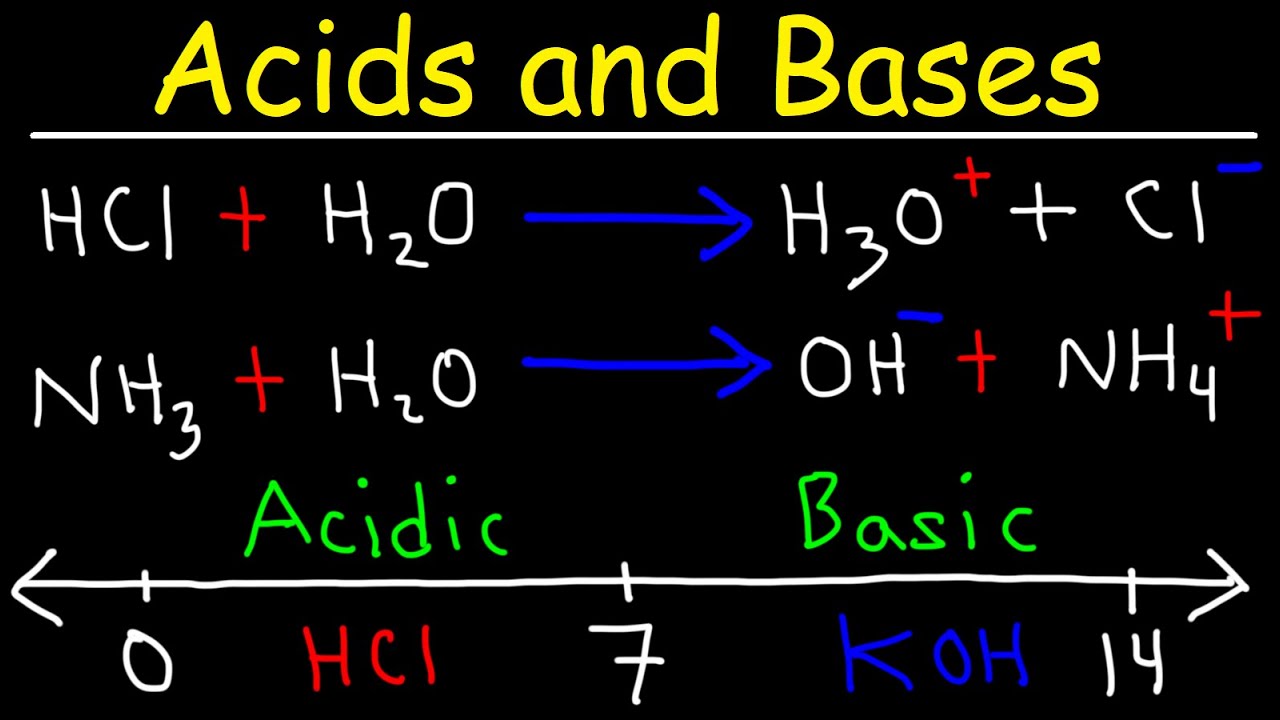
- 🌊 Water acts as a medium for acid-base reactions, accepting protons from acids and donating them to bases, facilitating neutralization.
- 🏦 Water is likened to a resilient and fair banker for acids and bases, always available and not charging interest, essential for the functioning of the chemical economy.
Q & A
What are some common applications of acids and bases?
-Acids and bases are used in the production of foods, soaps and detergents, fertilizers, explosives, dyes, plastics, and pesticides. They are also found in everyday items like the acid in our stomachs and the basic nature of our blood.
How were acids and bases defined before the understanding of atoms and molecules?
-Before the concept of atoms and molecules, acids and bases were defined by their properties. Acids were known to taste sour and corrode metal, while bases felt slippery and could counteract the effects of acids.
What is the role of protons and electrons in the interaction of molecules dissolved in water?
-When molecules dissolve in water, they interact by exchanging protons (hydrogen ions) and electrons with their surroundings. Depending on the molecule's composition, it may be willing to donate or accept these particles, affecting its charge.
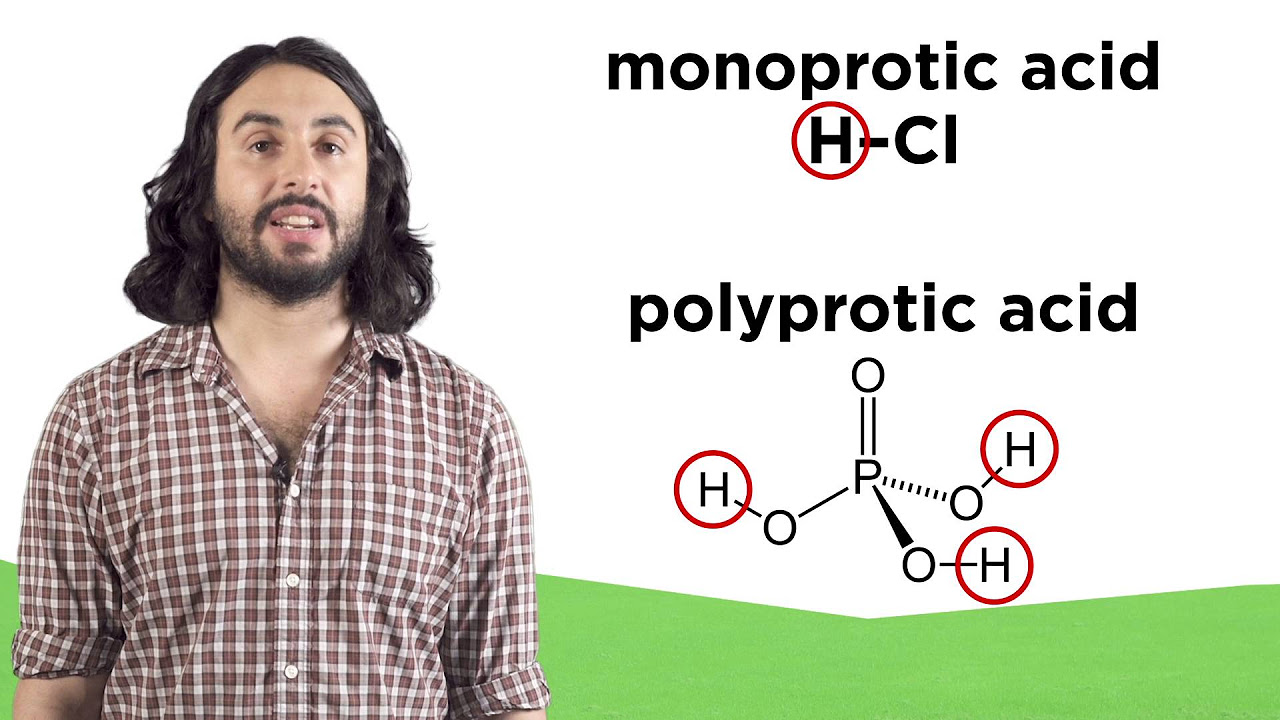
What is the difference between strong acids and weak acids?
-Strong acids are very aggressive in donating their protons to the surrounding water molecules. In contrast, weak acids may only donate a few of their protons or accept a few protons from water, with most of their molecules remaining unchanged.
How do strong bases interact with water?
-Strong bases are eager to accept a proton from water, often removing one even when water has already donated two protons. This makes strong bases highly effective at increasing the pH of a solution.
What is the equilibrium point in the context of weak acids and bases in water?
-The equilibrium point refers to a state where a small fraction of the weak acid or base molecules have exchanged protons with water. This balance is dynamic, and the molecules can revert to their original state, maintaining a certain concentration of molecules in the solution.
Can you provide examples of weak acids and bases from everyday life?
-An example of a weak acid is acetic acid, found in vinegar, which is responsible for its pungent smell. A common weak base is ammonia, often used as a cleaning agent for glass to provide a streak-free shine.
How does water act as a medium for acid-base reactions?
-Water can act as both an acid and a base, accepting protons from acids or donating protons to bases. This dual role allows water to facilitate acid-base reactions and maintain a balance, functioning like a molecular ATM.
What is neutralization in the context of acid-base chemistry?
-Neutralization occurs when an equal amount of acid and base react, resulting in a net effect of zero change to water's proton balance. This typically produces a salt and water as the products of the reaction.
How does the script describe water's role in acid-base chemistry?
-The script likens water to a resilient and fair banker for acids and bases. It is always available, does not charge interest, and will not 'foreclose' on molecules, highlighting water's essential and non-judgmental role in facilitating acid-base reactions.
What can we infer about the importance of acids and bases in our daily lives from the script?
-The script emphasizes that acids and bases are ubiquitous in our daily lives, from the food we eat to the cleaning products we use. They play crucial roles in both natural processes and human-made products, highlighting their fundamental importance in our world.
Outlines
🌟 Introduction to Acids and Bases
This paragraph introduces the ubiquitous nature of acids and bases in our daily lives, highlighting their applications in various industries such as food, cleaning products, agriculture, and more. It emphasizes the fundamental role of acids and bases in our biological systems, from the acidity of our stomachs to the basic nature of our blood and the amino acids that make up our proteins. The historical context of acids and bases is also touched upon, noting that their definitions were based on observable properties long before the understanding of atoms and molecules. The paragraph sets the stage for a deeper exploration of acid-base chemistry by discussing the interaction of molecules with their environment through the exchange of protons and electrons, establishing the groundwork for understanding the behavior of acids and bases in aqueous solutions.
Mindmap
Keywords
💡Acids and Bases
💡Protons and Electrons
💡Molecular Level
💡Ancient Greek
💡Strong Acids and Bases
💡Weak Acids and Bases
💡Water
💡Neutralization
💡Chemical Economy
💡Amino Acids
💡Genetic Code
Highlights
Acids and bases are ubiquitous in our daily lives and are used in a wide range of products such as foods, soaps, detergents, fertilizers, explosives, dyes, plastics, and pesticides.
The human body exhibits natural acidity and alkalinity with stomachs being very acidic and blood slightly basic.
Amino acids, the building blocks of proteins, and the bases in our genetic code (A, T, C, G) are fundamental to life.
Ancient Greeks defined acids and bases by their observable properties before the concept of atoms or molecules was established.
Molecules in water interact by exchanging protons (hydrogen ions) and electrons with their surroundings.
The willingness of a molecule to donate or accept protons or electrons determines its acidic or basic nature.
Protons are positively charged and electrons are negatively charged, influencing how molecules interact in chemical reactions.
Molecules that aggressively donate protons are classified as strong acids, such as those that can donate multiple protons to water.
Strong bases are compounds eager to accept protons, often taking them from water molecules.
Weak acids and bases partially donate or accept protons from water but most of their molecules remain unchanged in water.
Even though they are called weak, substances like vinegar and ammonia are still potent in their acidic and basic properties, respectively.
Water acts as a universal solvent and mediator in acid-base reactions, facilitating the exchange of protons.
Neutralization occurs when the effects of an acid and a base on water's proton balance cancel each other out.
Some molecules can exhibit acidic or basic properties without the presence of water.
Water's role as a resilient and fair mediator for acids and bases is essential for chemical reactions and is always available without negative consequences.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: