Ch#24 | Lec#1|| Combustion Analysis +Numericals #Analytical Chemistry Class Class 12
TLDRThe video script discusses the importance of analytical and numerical methods in chemistry, specifically focusing on combustion analysis. It emphasizes the demand for understanding these concepts, particularly among students. The script outlines the process of determining the percentage of elements in a compound through combustion, highlighting the steps involved in qualitative and quantitative analysis. It also touches on the limitations of combustion analysis, noting that it is applicable only to organic compounds containing carbon, hydrogen, and oxygen. The video aims to clarify these complex concepts, making them more accessible and engaging for the audience.
Takeaways
- 📚 The lecture focuses on analytical and numerical methods in chemistry, specifically for students preparing for exams.
- 🧪 The importance of understanding both qualitative and quantitative analysis in chemistry is emphasized for solving complex problems.
- 🔍 The concept of combustion analysis is introduced as a crucial topic for exams, involving the identification of elements in compounds through classical methods and modern techniques like spectroscopy.
- 🔥 The process of combustion analysis involves burning an organic compound in a combustion tube and separating the resulting carbon dioxide and water for further analysis.
- 📈 The calculation of percentage composition of elements in a compound is demonstrated using the mass of the compound and the mass of the combustion products.
- 🌟 The use of the empirical formula to represent the simplest whole number ratio of atoms in a compound is discussed, as well as its limitations when other elements are present.
- 📝 The steps to determine the molecular formula of an organic compound are outlined, including finding the percentage of each element, the mass of each element, and the atomic ratio.
- 🤔 The script highlights the challenge students face in understanding and applying these concepts, suggesting the need for practice and proper methodology.
- 📊 The practical application of these concepts is demonstrated through examples, including the calculation of the empirical formula from given data.
- 🎓 The lecture encourages students to practice and understand the concepts thoroughly to solve numerical problems in exams effectively.
- 🔄 The process of converting the empirical formula to the molecular formula by considering the molar mass and adding the number of molecules is briefly explained.
Q & A
What is the main topic discussed in the script?
-The main topic discussed in the script is Analytical Chemistry, specifically focusing on combustion analysis and the process of determining the empirical and molecular formulas of organic compounds.
What are the two types of analysis mentioned in the script?
-The two types of analysis mentioned in the script are qualitative analysis and quantitative analysis.
What is the purpose of combustion analysis in chemistry?
-The purpose of combustion analysis is to determine the chemical composition of a substance, specifically the amounts of carbon, hydrogen, and oxygen present in an organic compound.
How is the mass of the compound determined in combustion analysis?
-The mass of the compound is determined by the known mass of the organic compound that is burned in the combustion tube.
What are the products of complete combustion of an organic compound?
-The products of complete combustion of an organic compound are carbon dioxide (CO2) and water (H2O).
How is the percentage of carbon in the compound determined?
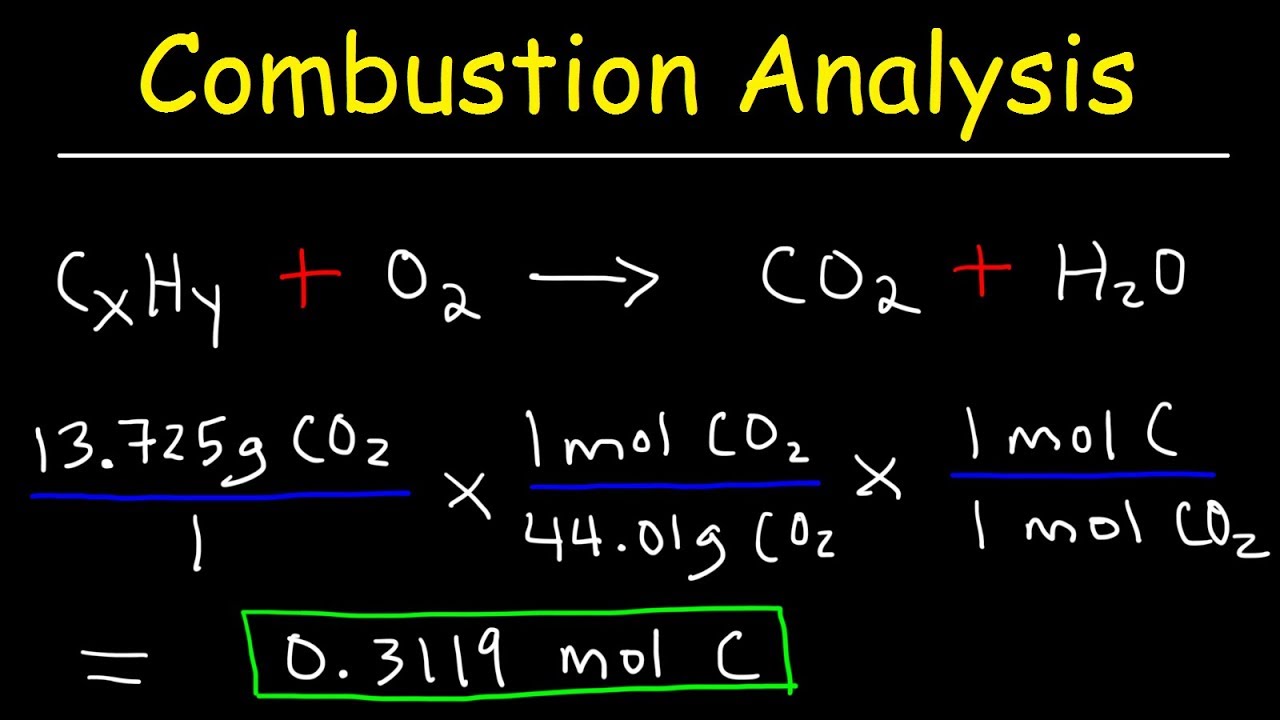
-The percentage of carbon is determined by the mass of carbon dioxide (CO2) produced, using the formula: % Carbon = (mass of CO2 / mass of compound) * 100.
What is the role of magnesium perchlorate in the combustion process?
-Magnesium perchlorate acts as a drying agent in the combustion process, absorbing water (H2O) produced during combustion to ensure accurate measurement of the other combustion products.
What is the significance of the empirical formula in chemistry?
-The empirical formula represents the simplest whole-number ratio of atoms in a compound, providing a basic understanding of its composition without specifying the number of molecules or the molecular weight.
How is the molecular formula derived from the empirical formula?
-The molecular formula is derived from the empirical formula by determining the molar mass of the compound and then finding the whole-number multiple that will give the actual mass of each element in the compound.
What is the difference between the empirical and molecular formulas?
-The empirical formula represents the simplest ratio of elements in a compound, while the molecular formula represents the actual number of atoms of each element in a molecule of the compound, reflecting the compound's true molecular structure.
Why is it important to know the molecular formula of a compound?
-Knowing the molecular formula is important because it provides detailed information about the compound's structure and composition, which is essential for understanding its properties, reactivity, and potential applications.
Outlines
📚 Introduction to Analytical and Numerical Analysis
The paragraph introduces the topic of analytical and numerical analysis in chemistry, emphasizing its importance and relevance in solving problems related to children's chapters. The speaker discusses the demand for understanding these methods, especially for students, and outlines the plan to explain the concepts in an accessible way to help them grasp the subject better. The introduction sets the stage for a detailed discussion on qualitative and quantitative analysis, setting up the foundation for further exploration of the topic.
🔍 Understanding Combustion Analysis
This paragraph delves into the specifics of combustion analysis, a classical method used to determine the percentage composition of elements in an organic compound. The speaker explains the process of combustion, where the compound is burned in a presence of oxygen, resulting in the formation of carbon dioxide and water. The paragraph outlines the steps to calculate the mass of CO2 and H2O produced, and how to use these values to determine the percentage of carbon, hydrogen, and oxygen in the original compound. The explanation is detailed, providing a clear understanding of the combustion analysis technique.
📈 Numerical and Molecular Formula Determination
The focus of this paragraph is on the methods for finding the empirical and molecular formulas of organic compounds. The speaker explains the steps involved in determining the percentage of each element in the compound, which is crucial for calculating the empirical formula. The paragraph also touches on the concept of atomic mass and how it is used in these calculations. The speaker encourages students to practice these techniques, emphasizing their importance in understanding and solving numerical problems related to chemical compounds.
🧪 Practical Approach to Numerical Analysis
This paragraph provides a practical approach to numerical analysis, guiding students through the process of calculating the empirical formula of a compound. The speaker uses a hypothetical example of an organic compound containing carbon, hydrogen, and oxygen, and walks through the steps of determining the percentages of each element, calculating the mass of elements, and finally, deriving the empirical formula. The explanation is detailed, offering a clear methodology that students can follow to solve similar problems.
📊 Empirical Formula Calculation
The paragraph is centered around the calculation of the empirical formula of a compound. The speaker provides a detailed explanation of how to use the given percentages of carbon, hydrogen, and oxygen to calculate the mass of these elements in the compound. The process involves using the atomic masses of the elements and performing calculations to find the ratio of atoms in the compound. The speaker emphasizes the importance of this step in understanding the composition of the compound and provides a clear, step-by-step guide for students to follow.
🌟 Finalizing the Molecular Formula
In this paragraph, the speaker concludes the process of determining the molecular formula from the empirical formula. The explanation involves using the empirical formula to find the molecular mass and then calculating the actual molecular formula. The speaker provides a clear example, demonstrating how to solve the problem and verify the results using a calculator. The paragraph emphasizes the importance of this final step in fully understanding the chemical structure of the compound.
Mindmap
Keywords
💡Combustion Analysis
💡Empirical Formula
💡Molecular Formula
💡Quantitative Analysis
💡Elemental Detection
💡Spectroscopy
💡Mole Concept
💡Atomic Mass
💡Qualitative Analysis
💡Percentage Composition
💡Detector
Highlights
Introduction to Analytical Chemistry and its increasing demand, especially among students.
The importance of understanding the theory behind the chapters, particularly for numerical problems.
Explaining the concept of qualitative analysis, including the identification of different elements present in a compound.
Quantitative analysis and its significance in determining the amount of different elements in a compound.
The process of combustion analysis, a classic method for determining carbon and hydrogen in organic compounds.
The role of modern techniques like spectroscopy in analytical chemistry.
The challenge of solving numerical problems in combustion analysis and the need to understand the concepts behind them.
The formula for calculating the percentage of carbon in a compound using combustion analysis.
The calculation of hydrogen percentage in a compound through analytical methods.
The determination of oxygen percentage by difference in the compound analysis.
The importance of empirical and molecular formulas in understanding the composition of organic compounds.
The steps involved in finding the empirical formula of a compound, including percentage calculation and mass determination.
The process of converting an empirical formula to a molecular formula using molar mass.
The application of analytical chemistry techniques in identifying elements beyond carbon, hydrogen, and oxygen in organic compounds.
The limitations of combustion analysis in determining the molecular formula of compounds with elements other than carbon, hydrogen, and oxygen.
The use of spectroscopy as a modern technique for a more comprehensive analysis of organic compounds.
Transcripts
Browse More Related Video
Introduction to Combustion Analysis, Empirical Formula & Molecular Formula Problems

Lec-42 I Qualitative analysis of organic substance_CT I Applied chemistry I Chemical engineering
Balancing Combustion Reactions

CH403 0 The Analytical Process

Types of Chemical Reactions

Chapter 0: What is Analytical Chemistry | CHM 214 | 001
5.0 / 5 (0 votes)
Thanks for rating: