Exothermic and Endothermic vs Exergonic and Endergonic (simplified)
TLDRThe video script discusses the concepts of endothermic and exothermic processes, differentiating them by the type of energy change involved. It explains that endothermic processes involve a change in potential energy, while exothermic processes involve a change in free energy, which is the energy available to do work. Using the example of hydrocarbon combustion, the script illustrates how energy is released in the form of heat, and how the Gibbs free energy equation (ΔG = ΔH - TΔS) helps determine the amount of energy that can be harnessed for work, taking into account the energy lost due to entropy.
Takeaways
- 🔄 The main difference between endothermic and exothermic processes is the direction of energy flow, with endothermic processes absorbing energy and exothermic processes releasing it.
- ⚛️ Endothermic reactions involve a change in potential energy, while endergonic reactions involve a change in free energy, which is the energy available to do work.
- 🔥 The combustion of a hydrocarbon, such as methane, is an example of an exothermic reaction where heat is released and can be used as a product or a reactant.
- 📉 In any chemical process, some energy is inevitably lost due to entropy, which means that no reaction is 100% efficient.
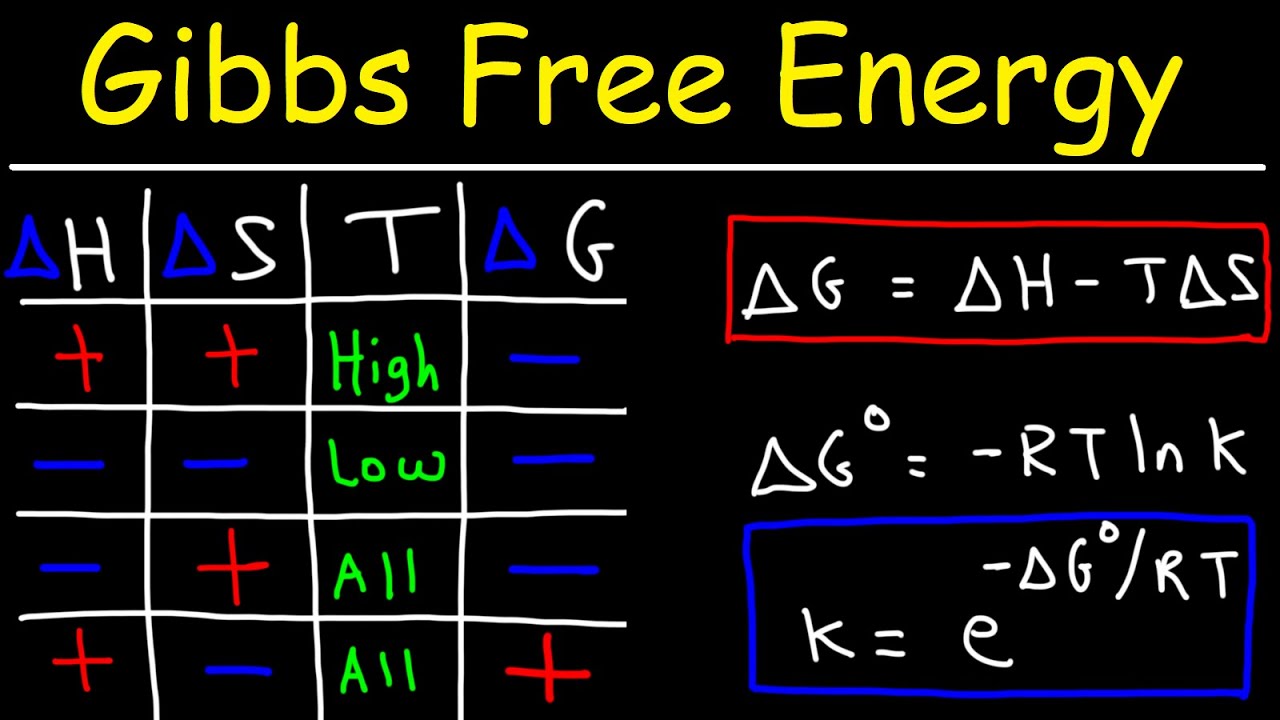
- 📊 Gibbs free energy (ΔG) is a measure of the energy available to do work in a reaction, calculated as ΔG = ΔH - TΔS, where ΔH is the change in enthalpy and ΔS is the change in entropy.
- 🌡️ Temperature is a factor in the Gibbs free energy equation to maintain dimensional consistency and to account for the effect of temperature on the reaction.
- 🔄 The concept of potential energy change in a reaction is crucial for understanding the energy dynamics and the work that can be extracted from a process.
- 💡 The script emphasizes the importance of understanding the energy changes in chemical reactions, especially in terms of potential energy and free energy.
- 🔧 The practical application of these concepts is highlighted by the example of using the heat released in combustion reactions to perform work.
- 📚 The script serves as a teaching tool to clarify common misconceptions between endothermic/exothermic and endergonic/exergonic processes.
Q & A
What is the main difference between endothermic and exothermic processes?
-The main difference lies in the direction of energy flow. In endothermic processes, energy is absorbed, leading to an increase in potential energy. In exothermic processes, energy is released, resulting in a decrease in potential energy.
What does the term 'free energy' refer to in the context of the script?
-Free energy refers to the amount of energy available to do work. It is the energy that can be harnessed from a process, such as the combustion of a hydrocarbon, after accounting for energy losses due to entropy.
How does the combustion of a hydrocarbon, like methane, illustrate the concepts of potential and free energy?
-The combustion of methane with oxygen produces CO2 and H2O while releasing a significant amount of heat. This release of heat represents a decrease in potential energy. However, not all the energy released can be used to do work due to entropy losses, which is accounted for in the concept of free energy.
What is the Gibbs free energy and how is it calculated?
-Gibbs free energy is a thermodynamic potential that measures the maximum reversible work that can be done by a system at constant temperature and pressure. It is calculated using the equation ΔG = ΔH - TΔS, where ΔH is the change in enthalpy, T is the temperature, and ΔS is the change in entropy.
Why can't we get a 100% yield in chemical reactions?
-Due to the second law of thermodynamics, processes are never 100% efficient as some energy is always lost to entropy. This means that in chemical reactions, it is impossible to capture all the energy released and convert it entirely into useful work.
What does the script imply about the relationship between potential energy, heat, and work in a chemical reaction?
-The script implies that potential energy can be converted into heat during a chemical reaction, and this heat can then be harnessed to do work. However, the amount of work that can be done is limited by the energy lost to entropy, as reflected in the calculation of Gibbs free energy.
How does the script's explanation of energy changes help in understanding chemical reactions?
-By understanding the changes in potential and free energy, one can predict the spontaneity and feasibility of a chemical reaction. It also provides insight into the energy available for work and the energy lost due to entropy, which is crucial for optimizing industrial processes and understanding reaction mechanisms.
What is the significance of the equation ΔG = ΔH - TΔS in the context of the script?
-This equation is significant as it quantifies the amount of energy available for work after accounting for entropy. It is a fundamental equation in thermodynamics that helps in determining whether a reaction is spontaneous and how much work can be extracted from it.
How does the concept of entropy affect the energy available for work in a process?
-Entropy represents the degree of disorder or randomness in a system. In the context of the script, it is the reason why not all energy released in a process can be converted into work. Some energy is always lost to increase the entropy of the system, reducing the amount of usable work energy.
What is the role of temperature in the Gibbs free energy equation?
-Temperature plays a crucial role in the Gibbs free energy equation as it affects the magnitude of the entropy term (TΔS). Higher temperatures can increase the impact of entropy on the total free energy, thus influencing the spontaneity of a reaction and the amount of energy available for work.
How can the script's explanation of energy changes be applied to real-world scenarios?
-The principles of energy changes discussed in the script are fundamental to understanding energy conversions in various real-world applications, such as combustion engines, energy production, and chemical manufacturing. They help in designing efficient processes that minimize energy loss and maximize work output.
Outlines
🔬 Understanding Endothermic and Exothermic Processes
This paragraph discusses the concepts of endothermic and exothermic processes, highlighting the confusion that students often face due to their similar nature. It explains that in both processes, energy is involved, either entering or leaving the system. The key difference lies in the type of energy change, with endothermic or exothermic processes involving a change in potential energy. The paragraph further clarifies that in endergonic and exergonic processes, there is a change in free energy, which is the energy available to do work. An example of the combustion of a hydrocarbon is used to illustrate these concepts, emphasizing the release of heat and the potential to do work, while also acknowledging the role of entropy and the impossibility of achieving a 100% yield in chemical processes.
Mindmap
Keywords
💡endothermic
💡exothermic
💡potential energy
💡free energy
💡combustion
💡hydrocarbon
💡entropy
💡Gibbs free energy
💡reaction yield
💡thermodynamics
💡chemical reaction
Highlights
Endothermic and exothermic processes involve energy transfer.
Endergonic and exergonic processes involve a change in energy types.
In endothermic and endergonic processes, potential energy changes.
In exothermic and exergonic processes, free energy is involved.
Free energy is the energy available to do work.
Combustion of hydrocarbons, like methane, is an example of an exothermic and exergonic process.
Heat can be considered a product in exothermic reactions, similar to a reactant.
Potential energy decreases in exothermic reactions, releasing energy as heat.
Not all released energy can be used for work due to entropy.
Gibbs free energy measures the energy available for work, accounting for entropy.
The equation ΔG = ΔH - TΔS calculates Gibbs free energy.
ΔH represents the change in enthalpy, or total energy change.
ΔS is the change in entropy, representing energy lost to disorder.
Temperature is included in the Gibbs free energy equation for dimensional consistency.
Understanding these concepts is crucial for applications in chemistry and energy management.
The relationship between potential and free energy is fundamental to reaction spontaneity and work output.
This explanation provides a clear framework for comprehending energy transformations in chemical processes.
Transcripts
Browse More Related Video

Endothermic and Exothermic Reactions

18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemistry

GCSE Chemistry - Exothermic and Endothermic Reactions #43
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K

Gibbs free energy and spontaneity | Chemistry | Khan Academy

Endergonic, exergonic, exothermic, and endothermic reactions | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: