5.3 Limiting Reactant Calculations | High School Chemistry
TLDRIn this chemistry lesson, Chad from Chad's Prep explains the concept of limiting reagents, a fundamental topic in stoichiometry. He uses the analogy of building bicycles to simplify the idea, then applies it to chemical reactions, specifically the production of ammonia. Chad guides students through the process of identifying limiting reagents, calculating theoretical yields, and understanding percent yields. The lesson aims to demystify these abstract concepts, making them relatable and accessible for students, and encourages practice with both simple and complex numbers.
Takeaways
- 🧪 Limiting reagent calculations are fundamental to stoichiometry and a memorable part of high school chemistry.
- 🔍 The concept of limiting reagents is not difficult but requires understanding ratios and applying them to chemical compounds.
- 🚲 The script uses a tangible example of building bicycles to illustrate the concept of limiting reagents, making it easier to grasp.
- 📚 Theoretical yield and percent yield calculations are integral parts of limiting reagent calculations, essential for understanding chemical reactions.
- 📈 Dimensional analysis is a key technique used in limiting reagent calculations, involving the use of ratios to convert between different units.
- 🔢 The balanced chemical equation provides the ratio of reactants and products but does not indicate the actual amounts available.
- ⚖️ Converting grams to moles is often the first step in limiting reagent problems, using the molar mass from the periodic table.
- 🔄 The limiting reagent is the reactant that will be completely consumed first in a reaction, determining the maximum amount of product that can be formed.
- 📉 Once the limiting reagent is determined, the theoretical yield can be calculated based on the amount of the limiting reactant.
- 📊 Percent yield is calculated by comparing the actual yield (the amount of product actually produced) to the theoretical yield (the maximum possible amount).
- 📘 Practice with both 'nice' and complex numbers is crucial for mastering limiting reagent calculations and applying them to various chemistry problems.
Q & A
What is the concept of limiting reagents in chemistry?
-In chemistry, limiting reagents are reactants that are completely consumed in a chemical reaction and determine the maximum amount of product that can be formed. The limiting reagent is the one that runs out first during the reaction.
Why is it important to understand limiting reagents in stoichiometry?
-Understanding limiting reagents is crucial in stoichiometry because it helps determine the theoretical yield of a reaction. It allows chemists to calculate how much product can be produced from a given amount of reactants.
What is the relationship between the number of frames and tires in the bicycle example?
-In the bicycle example, every one frame requires two tires to make a bicycle. This 1:2 ratio is analogous to the mole-to-mole ratios in chemical reactions.
How do you determine the limiting reagent when you have more than one reactant?
-To determine the limiting reagent, you compare the mole-to-mole ratios of the reactants from the balanced chemical equation. The reactant that would be completely consumed first, based on these ratios, is the limiting reagent.
What is the significance of the theoretical yield in chemical reactions?
-The theoretical yield is the maximum amount of product that can be produced from a reaction, assuming 100% efficiency and no side reactions. It is calculated based on the limiting reagent and is used as a benchmark to compare with the actual yield obtained in a laboratory.
How does the concept of a balanced chemical equation help in determining the limiting reagent?
-A balanced chemical equation provides the mole-to-mole ratios of reactants and products. These ratios are used to determine how much of each reactant is needed to completely consume the limiting reagent, thereby identifying the limiting reagent in a reaction.
What is the difference between the actual yield and the theoretical yield?
-The actual yield is the amount of product actually produced in a reaction, which can be less than the theoretical yield due to inefficiencies, side reactions, or experimental errors. The theoretical yield is the maximum amount of product that could be produced if all reactants were perfectly converted into products.
How do you calculate the percent yield of a reaction?
-The percent yield is calculated by dividing the actual yield by the theoretical yield and multiplying by 100. It expresses the efficiency of a reaction as a percentage of the maximum possible yield.
Why is it necessary to convert grams to moles in stoichiometry calculations?
-Grams to moles conversion is necessary because the coefficients in a balanced chemical equation represent mole-to-mole ratios, not gram-to-gram ratios. Converting grams to moles allows for accurate stoichiometric calculations based on these ratios.
What is the role of molar mass in converting grams to moles?
-Molar mass is the mass of one mole of a substance. It is used to convert grams to moles by dividing the mass of the substance (in grams) by its molar mass. This conversion is essential for stoichiometry calculations involving balanced chemical equations.
Outlines
🚲 Introduction to Limiting Reagents and Theoretical Yield
The script introduces the concept of limiting reagents and theoretical yield, central to stoichiometry in chemistry. The instructor, Chad, uses a relatable analogy of building bicycles from recycled parts to explain these concepts. Each bicycle requires two tires and one frame, illustrating the need for specific ratios of reactants in chemical reactions. Chad emphasizes the importance of understanding these calculations, which form a memorable part of high school chemistry. The lesson aims to make science understandable and enjoyable, with Chad's Prep offering weekly lessons throughout the 2020-21 school year.
🔍 Understanding Mole Ratios and Limiting Reagents
This paragraph delves into the specifics of mole ratios and how they determine the limiting reagent in a chemical reaction. The example of ammonia (NH3) production from nitrogen (N2) and hydrogen (H2) is used to illustrate the concept. The balanced chemical equation shows that one mole of N2 reacts with three moles of H2 to produce two moles of NH3. Chad explains that the balanced equation provides the ratio of reactants needed but does not indicate the actual amounts available. The limiting reagent is identified by comparing the mole ratios of the reactants, not by the total mass or the amount initially present.
📚 Calculation Techniques for Limiting Reagents
The script outlines the process for determining the limiting reagent and calculating the theoretical yield. Chad provides a step-by-step method involving converting grams to moles using molar masses, applying mole ratios from the balanced chemical equation, and then determining which reactant limits the reaction. He warns against the common mistake of assuming the reactant in excess based on grams alone, emphasizing the importance of converting to moles and considering the reaction ratio. The example of combining 112 grams of nitrogen and 30 grams of hydrogen is used to demonstrate the calculation process.
🧪 Application of Limiting Reagent Concepts in Ammonia Production
This section applies the previously discussed concepts to calculate the theoretical yield of ammonia when given specific amounts of nitrogen and hydrogen. Chad explains how to convert the limiting reactant (in this case, nitrogen) into moles of ammonia using the coefficients from the balanced chemical equation. He also discusses the theoretical yield, which is the maximum amount of product that can be produced if the reaction goes to completion without any side reactions. The example calculation shows that from the limiting reactant, 136 grams of ammonia could theoretically be produced.
📉 Calculating Excess Reactant and Percent Yield
The final paragraph addresses how to calculate the amount of excess reactant left after the reaction and the percent yield of the product. Chad uses the example of 112 grams of N2 and 30.0 grams of H2 reacting to form 102 grams of ammonia to illustrate the calculation of percent yield. He explains that the percent yield is determined by dividing the actual yield by the theoretical yield and multiplying by 100. The example results in a 75 percent yield, indicating that not all reactants were converted into the desired product. Chad advises practicing these calculations with both 'nice' and more complex numbers to solidify understanding.
Mindmap
Keywords
💡Limiting Reagent
💡Stoichiometry
💡Theoretical Yield
💡Percent Yield
💡Molar Mass
💡Balanced Chemical Equation
💡Mole-to-Mole Ratio
💡Reagent in Excess
💡Chemical Reaction
💡Conversion Factor
Highlights
Limiting reagent calculations are foundational in stoichiometry and a memorable high school chemistry lesson.
Chad introduces his high school chemistry playlist, posting weekly lessons throughout the 2020-21 school year.
The concept of limiting reagents is introduced using a tangible example of building bicycles, requiring two tires and one frame.
The importance of understanding ratios in chemical reactions is emphasized, not just the amounts of reactants.
The example of ammonia (NH3) production is used to illustrate the concept of balanced chemical reactions and molar ratios.
It's clarified that a balanced chemical reaction only provides ratios, not the actual amounts of reactants or products.
The process of converting grams to moles is crucial in stoichiometry, especially when dealing with diatomic molecules like N2 and H2.
The concept of theoretical yield is introduced, explaining the maximum amount of product that can be produced in a reaction.
The difference between theoretical yield and actual yield is discussed, highlighting the impact of side reactions in real-world chemistry.
The method for determining the limiting reagent is explained, focusing on the ratio of reactants to their coefficients in the balanced equation.
A step-by-step process for calculating the amount of product formed from the limiting reagent is outlined.
The calculation of excess reagent and its remaining amount after the reaction is detailed, emphasizing the subtraction of used reactant from the initial amount.
The percent yield is defined and calculated, comparing the actual yield to the theoretical yield to determine efficiency.
The importance of practicing limiting reagent calculations with both simple and complex numbers is stressed for a thorough understanding.
Chad encourages viewers to like, share, and subscribe for more educational content and to check out his premium course for additional study materials.
Transcripts
Browse More Related Video

Stoichiometry: Limiting Reactant, Left Over Excess Reactant, Percent Yield | Study Chemistry With Us
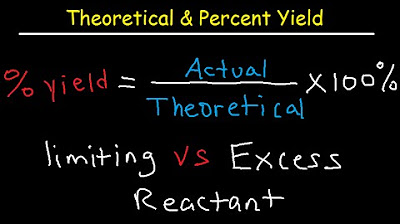
Theoretical, Actual, Percent Yield & Error - Limiting Reagent and Excess Reactant That Remains
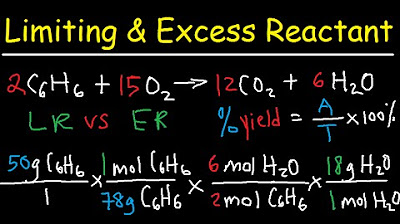
Stoichiometry - Limiting & Excess Reactant, Theoretical & Percent Yield - Chemistry

Most Common Chemistry Final Exam Question: Limiting Reactants Review

AP Chemistry Unit 4 Review: Chemical Reactions

16.6 Acidity and Basicity of Salts | General Chemistry
5.0 / 5 (0 votes)
Thanks for rating: