Most Common Chemistry Final Exam Question: Limiting Reactants Review
TLDRIn this educational video, the host guides viewers through the concept of limiting reactants in chemistry, using a relatable coffee-making analogy to illustrate the idea. They explain how to determine the limiting reactant, calculate theoretical yield, and understand the difference between theoretical and actual yield, including how to compute percent yield. The video aims to prepare students for common final exam questions on this topic, offering step-by-step instructions and practice problems to solidify the concepts.
Takeaways
- 📝 Limiting Reactant: The reactant that produces the least amount of product in a chemical reaction, determining the theoretical yield.
- 🔍 Coffee Analogy: The concept of limiting reactant is explained using coffee ingredients to illustrate the idea of ingredients limiting the final product.
- 🧪 Stoichiometry: The process of determining the limiting reactant involves converting grams of reactants to moles, using molar masses, and applying mole ratios from the balanced chemical equation.
- 📉 Theoretical Yield: The maximum amount of product that can be produced, based on the limiting reactant.
- 🚫 Excess Reactant (ER): The reactant that remains after the reaction is complete, not being fully consumed.
- ⚖️ Percent Yield: A measure of how close the actual yield of a reaction is to the theoretical yield, indicating the efficiency of the reaction.
- 🔄 Conversion Process: To find the limiting reactant, convert grams of each reactant to the desired product using stoichiometry, and compare the amounts.
- 📉 Actual Yield: The amount of product actually produced in a reaction, which can be less than the theoretical yield due to experimental errors.
- 📌 Mole Ratio: The balanced chemical equation provides the mole ratio necessary to convert between reactants and products.
- 📚 Practice Importance: The script emphasizes the importance of practicing stoichiometry problems to understand and apply the concepts effectively.
- 🔗 Additional Resources: The video suggests using detailed notes on limiting reactants for further practice, available on the instructor's website.
Q & A
What is a limiting reactant?
-A limiting reactant is the reactant that is completely consumed in a chemical reaction and determines the maximum amount of product that can be formed. It is the ingredient that produces the least amount of product in a reaction.
How can you determine the limiting reactant in a chemical reaction?
-To determine the limiting reactant, you compare the amounts of each reactant that would theoretically produce the desired product. The reactant that produces the least amount of product is the limiting reactant.
What is the theoretical yield in a chemical reaction?
-The theoretical yield is the maximum amount of product that can be produced in a chemical reaction, based on the limiting reactant. It is the amount of product that would be formed if the reaction went to completion with 100% efficiency.
What is the difference between theoretical yield and actual yield?
-The theoretical yield is the maximum amount of product that could be produced in an ideal scenario, while the actual yield is the amount of product that is actually produced in a real-world experiment. The actual yield is often less than the theoretical yield due to inefficiencies and losses.
How do you calculate the percent yield of a reaction?
-Percent yield is calculated by dividing the actual yield by the theoretical yield and then multiplying by 100. It expresses the efficiency of a reaction as a percentage.
What does a high percent yield indicate about a reaction?
-A high percent yield indicates that the reaction is close to the theoretical yield, suggesting that the reaction was efficient and there were minimal losses or side reactions.
What is an excess reactant?
-An excess reactant is the reactant that remains after the limiting reactant has been completely consumed in a chemical reaction. It does not limit the amount of product formed.
How can you find out how much of an excess reactant is left after a reaction?
-To find out how much of an excess reactant is left, you start with the theoretical yield, convert it to moles of the product, then use stoichiometry to find the moles of the excess reactant used, convert that back to grams, and finally subtract this from the initial amount of the excess reactant.
What is the significance of stoichiometry in determining the limiting reactant?
-Stoichiometry is crucial in determining the limiting reactant because it allows you to convert the amounts of reactants to moles, use the balanced equation to find the mole ratio between reactants and products, and then convert back to grams to compare which reactant would produce the least amount of product.
Why is it important to understand limiting reactants in chemistry?
-Understanding limiting reactants is important because it helps in predicting the outcome of chemical reactions, optimizing the use of reactants, and minimizing waste in industrial processes. It is a fundamental concept in chemical calculations and reaction analysis.
Outlines
📚 Chemistry Final Exam Review: Limiting Reactants
The video script begins with a chemistry final exam review focusing on limiting reactants. The presenter uses a coffee-making analogy to explain the concept, comparing ingredients to reactants and the final cup of coffee to the product. The example involves determining which ingredient (beans or milk) limits the amount of coffee that can be made, based on their quantities. The limiting reactant is identified as the one that produces the least amount of product, and the theoretical yield is discussed as the amount of product the limiting reactant can produce. The presenter also introduces the terms 'excess reactant' and 'theoretical yield' and explains their relevance in chemical reactions.
🔍 Understanding Stoichiometry and Moles in Limiting Reactants
In the second paragraph, the script delves into the stoichiometry of chemical reactions, emphasizing the conversion from grams of reactants to moles, and then to the product using molar mass and mole ratios from a balanced chemical equation. The presenter guides viewers through the process of identifying the limiting reactant by comparing the theoretical amounts of product that can be produced from each reactant. The example involves calculating moles of iron from iron oxide and carbon monoxide, using their respective molar masses and the balanced equation to find the limiting reactant. The importance of understanding multiple ratios and molar mass in this process is highlighted.
📉 Determining the Limiting Reactant and Theoretical Yield
The third paragraph continues the discussion on limiting reactants, focusing on comparing the amounts of product that different reactants can theoretically produce. The presenter uses the results from the stoichiometry calculations to identify the limiting reactant, which is the one producing the least amount of product. The theoretical yield is reiterated as the amount of product the limiting reactant can produce. The script also touches on the irrelevance of excess reactant quantities beyond what is needed to produce the theoretical yield, reinforcing the concept with the coffee analogy.
🔄 Calculating Excess Reactant and Leftover Quantities
This paragraph introduces the concept of finding the excess reactant and how much of it is left over after the reaction. The presenter explains that the excess reactant is completely used up in the reaction, and the amount used can be calculated by starting from the theoretical yield and working back to the grams of the excess reactant. The formula for finding the leftover amount of the excess reactant is discussed, and the process involves subtracting the amount used in the reaction from the initial amount given. The coffee analogy is revisited to illustrate the concept of leftover reactants.
💯 Percent Yield and Its Significance in Chemistry
The final paragraph discusses the concept of percent yield, which measures the efficiency of a chemical reaction by comparing the actual yield to the theoretical yield. The presenter explains that percent yield indicates how close the actual experimental results are to the expected outcomes, with deviations suggesting errors such as spills or evaporation. The script also covers how to calculate percent yield and the implications of abnormal values, such as yields greater than 100% indicating contamination. The importance of understanding and practicing percent yield calculations for exams is emphasized.
Mindmap
Keywords
💡Limiting Reactant
💡Theoretical Yield
💡Excess Reactant
💡Stoichiometry
💡Molar Mass
💡Balanced Equation
💡Percent Yield
💡Actual Yield
💡Moles
💡Multiple Ratio
Highlights
Introduction to limiting reactants concept using a coffee-making analogy.
Explanation of limiting reactant as the ingredient that produces the least amount of product.
Illustration of theoretical yield as the amount of product the limiting reactant can produce.
Differentiation between limiting reactant and excess reactant in a chemical reaction.
Process of determining the limiting reactant through stoichiometry calculations.
Use of molar mass to convert grams of reactants to moles for stoichiometric calculations.
Importance of using the balanced chemical equation for mole ratio calculations.
How to find the theoretical yield from the limiting reactant in a chemical reaction.
Clarification on the irrelevance of excess reactant quantity beyond the limiting reactant's capacity.
Demonstration of the stoichiometric calculation process using an example with Fe2O3 and CO.
Explanation of how to calculate the amount of product a reactant can yield in a chemical reaction.
Method to determine the excess reactant and its leftover quantity after the reaction.
Introduction to the concept of percent yield in chemical reactions.
Explanation of percent yield as a measure of experimental accuracy compared to theoretical yield.
How to calculate percent yield using actual yield and theoretical yield.
Scenarios where percent yield might indicate experimental errors or contamination.
Method to find the actual yield from a given percent yield and theoretical yield.
Encouragement for students to practice limiting reactant problems for exam preparation.
Resource recommendation for detailed notes on limiting reactants for further study.
Transcripts
Browse More Related Video

Stoichiometry: Limiting Reactant, Left Over Excess Reactant, Percent Yield | Study Chemistry With Us
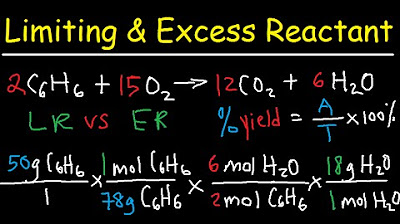
Stoichiometry - Limiting & Excess Reactant, Theoretical & Percent Yield - Chemistry

The Limiting Reactant Question That's Found on Most Final Exams | Study Chemistry With Us
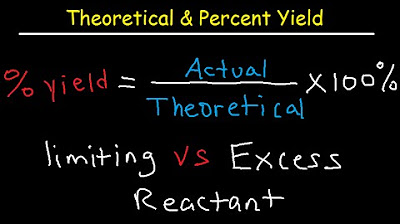
Theoretical, Actual, Percent Yield & Error - Limiting Reagent and Excess Reactant That Remains

How To Calculate The Percent Yield and Theoretical Yield

Practice Problem: Limiting Reagent and Percent Yield
5.0 / 5 (0 votes)
Thanks for rating: