AP Chemistry Unit 4 Review: Chemical Reactions
TLDRThis chemistry tutorial by Quran covers the challenging aspects of Unit 4, focusing on limiting reagents, stoichiometry, and types of chemical reactions including redox reactions. The video simplifies complex concepts like balancing equations and identifying limiting reagents by converting to moles. It also explains the difference between physical and chemical reactions, and delves into various reaction types like decomposition, synthesis, single and double replacement. The presenter uses relatable examples and mnemonics to make the content accessible, ensuring viewers grasp essential chemistry concepts.
Takeaways
- 🔍 The video covers the more challenging aspects of Unit 4 in chemistry, focusing on limiting reagents, stoichiometry, chemical reaction types, and redox reaction balancing.
- 📚 Basic stoichiometry is assumed to be known, as it's considered fundamental for understanding the material presented in the video.
- 🔢 Converting grams to moles is emphasized as a crucial step in solving stoichiometry problems, making the process simpler and more manageable.
- 🔄 The concept of limiting reagents is explained, with an example of a combustion reaction, highlighting how to determine which reactant limits the reaction.
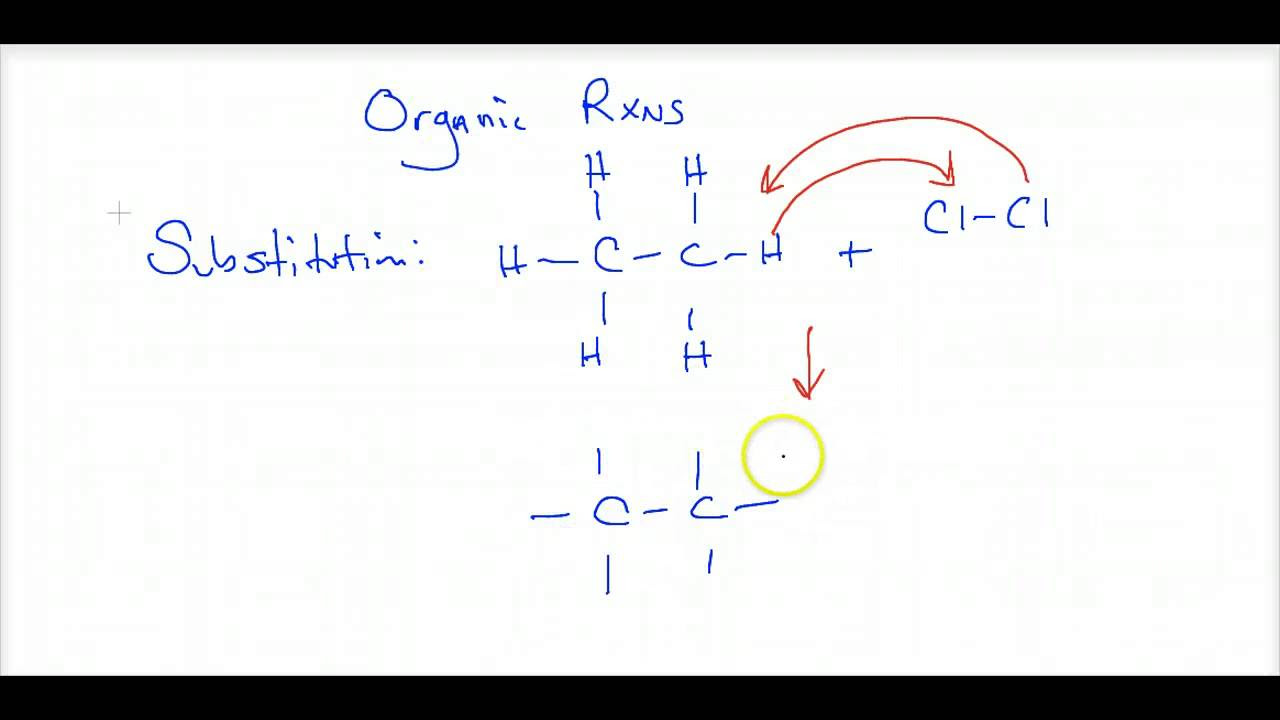
- ⚗️ The difference between physical and chemical reactions is clarified, with physical reactions not altering the composition of compounds, while chemical reactions involve bond breaking and forming.
- 🔍📈 The video introduces the types of chemical reactions: synthesis, decomposition, single replacement, double replacement, and redox reactions, each with distinct characteristics.
- 🧪 A method for solving stoichiometry problems without converting to moles is presented, using mass percent to find the mass of products in decomposition reactions.
- 🌐 The process of writing complete and net ionic equations for double replacement reactions, especially precipitation reactions, is detailed, showing how to simplify the reaction.
- 💧 The neutralization reaction is explained as a type of double replacement reaction, resulting in the formation of water and a salt, with the net ionic equation highlighting the essential components.
- 🔋 Redox reactions are described as involving the transfer of electrons, with a mnemonic 'OIL RIG' to remember oxidation (loss) and reduction (gain) of electrons.
- 🌀 Balancing redox reactions involves creating half-reactions for each element that changes oxidation state, ensuring electron conservation, and adjusting for oxygen and hydrogen balance.
Q & A
What is the main topic covered in the video script?
-The main topic covered in the video script is the more challenging aspects of Unit 4 in chemistry, including limiting reagents, stoichiometry, types of chemical reactions, and redox reaction balancing.
Why does the speaker mention they will skip basic stoichiometry?
-The speaker mentions skipping basic stoichiometry because they assume the viewers should already know how to do it, and if not, they would be at a significant disadvantage in understanding the rest of the content.
What is the first example of a chemical reaction given in the script?
-The first example of a chemical reaction given in the script is the combustion of methane (CH4) with oxygen to produce carbon dioxide (CO2) and water.
How does the speaker simplify the process of finding the limiting reagent?
-The speaker simplifies the process by emphasizing the importance of converting grams to moles and then comparing the mole ratios to determine the limiting reagent.
What is the difference between physical and chemical reactions as explained in the script?
-Physical reactions involve no change in the compounds themselves, meaning no bonds are broken or formed, like phase changes. Chemical reactions involve a change in the compounds, with bonds being broken or formed, resulting in the formation of new substances.
What is the purpose of mass percent in the context of the script?
-Mass percent is used in the script to find the mass of a particular element in a compound without having to convert to moles, which can simplify the calculation process in certain scenarios.
What are the different types of chemical reactions mentioned in the script?
-The different types of chemical reactions mentioned in the script are decomposition, synthesis, single replacement, double replacement, and redox reactions.
How does the speaker describe the process of balancing a redox reaction?
-The speaker describes balancing a redox reaction by first determining the oxidation states, splitting the reaction into half-reactions, ensuring the electrons cancel out, and then balancing the oxygen and hydrogen atoms.
What is the significance of the mnemonic 'OIL RIG' in the context of redox reactions?
-The mnemonic 'OIL RIG' stands for 'Oxidation Is Loss, Reduction Is Gain,' which helps remember that oxidation involves losing electrons (increasing positive charge) and reduction involves gaining electrons (increasing negative charge).
How does the speaker suggest dealing with the AP exam's emphasis on stoichiometry?
-The speaker suggests that when dealing with the AP exam's emphasis on stoichiometry, one should always convert to moles if in doubt, as it simplifies calculations and allows for the use of coefficients in reactions.
Outlines
🔍 Chemistry Unit 4 Overview
The speaker begins by addressing a previous statement about avoiding full unit walkthroughs but decides to cover Unit 4 due to its importance. The unit includes topics such as limiting reagents, stoichiometry, chemical reaction types, and redox reaction balancing. The speaker promises a detailed explanation of these concepts, starting with a typical combustion reaction example involving methane (CH4) and oxygen, emphasizing the importance of converting grams to moles for easier stoichiometry calculations. The paragraph also introduces the concept of limiting reagents and how to determine them through mole ratios and reaction extents.
📚 Stoichiometry and Chemical Reaction Types
This paragraph delves into the specifics of stoichiometry, explaining the process of converting mass to moles and using mole ratios to determine limiting reagents. The speaker uses an example with given masses of methane and oxygen to demonstrate the calculation. It also touches on the difference between physical and chemical reactions, highlighting that chemical reactions involve the breaking and forming of bonds, while physical reactions do not. The paragraph continues with an introduction to the types of chemical reactions: synthesis, decomposition, single replacement, double replacement, and redox reactions, with a brief mention of the mnemonic 'OIL RIG' to remember oxidation and reduction.
🧪 Balancing Redox Reactions and Precipitation
The speaker discusses the process of balancing redox reactions, starting with determining the oxidation states of elements involved. An example of a redox reaction is given, and the speaker explains how to split the reaction into half-reactions for balancing. The least common multiple method is introduced to balance the electrons transferred during the reaction. The paragraph also covers how to balance oxygen atoms by adding water (H2O) molecules and hydrogen ions (H+) to ensure the conservation of mass and charge. Additionally, the speaker briefly explains how to adjust the balanced equation for acidic and basic solutions.
🌟 Conclusion and Encouragement for Further Content
In the concluding paragraph, the speaker summarizes the key points of the video, emphasizing the importance of stoichiometry and the conversion to moles as a fundamental approach to solving chemical problems. The speaker encourages viewers to request more chemistry walkthroughs and reviews if they find the content helpful, highlighting the positive feedback received from previous videos as a motivation to continue creating educational content.
Mindmap
Keywords
💡Stoichiometry
💡Limiting Reagent
💡Combustion Reaction
💡Moles
💡Chemical Reactions
💡Decomposition Reaction
💡Synthesis Reaction
💡Single Replacement Reaction
💡Double Replacement Reaction
💡Precipitation Reaction
💡Redox Reaction
💡Net Ionic Equation
💡Neutralization Reaction
Highlights
Introduction to the concept of limiting reagent and stoichiometry in chemical reactions.
Explanation of why converting to moles simplifies stoichiometry calculations.
Demonstration of how to determine the limiting reagent in a combustion reaction.
Tutorial on calculating the amount of reactants left after a chemical reaction.
Clarification of the difference between physical and chemical reactions.
Overview of the types of chemical reactions: synthesis, decomposition, single replacement, double replacement, and redox.
Description of a technique to find the mass of product formed in decomposition reactions using mass percent.
Explanation of how to write and simplify ionic equations for double replacement reactions.
Discussion on the process of balancing redox reactions, including the method of half-reactions.
Technique for balancing redox reactions in both acidic and basic solutions.
The importance of converting to moles in stoichiometry and how to apply it to various chemical reactions.
The significance of understanding electron transfer in redox reactions and its impact on balancing equations.
How to identify and write net ionic equations for precipitation reactions.
The concept of neutralization reactions and how to write their ionic equations.
Practical tips for students preparing for the AP Chemistry exam, focusing on stoichiometry and reaction types.
Encouragement for viewers to request more chemistry walkthroughs and support for the channel.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: