How To Name Acids - The Fast & Easy Way!
TLDRThis educational video script teaches the process of naming acids by focusing on the rules for polyatomic and monoatomic ions. It demonstrates how to name common acids like sulfuric, hydrosulfuric, perchloric, and hypochlorous, and provides examples for acids with different polyatomic ions like nitrate and acetate. The script also explains how to derive the chemical formulas for acids such as phosphoric, phosphorous, carbonic, hydrobromic, and hydrocyanic, using the suffix rules to determine the correct nomenclature.
Takeaways
- 📝 Naming Acids: The script focuses on the rules for naming acids, especially when they contain polyatomic ions.
- 🔍 Polyatomic Ions: If an acid contains a polyatomic ion ending with 'ate', add 'ic' to form the name, and if it ends with 'ite', replace 'ite' with 'ous'.
- 🔬 Naming with 'ick': For polyatomic ions that end with 'ate', the suffix is changed to 'ic' and 'acid' is added to the end for the full name.
- 🌐 Suffix Replacement: Replace 'ate' with 'ic' for acids and 'ite' with 'ous' or 'ous acid' for different types of acids.
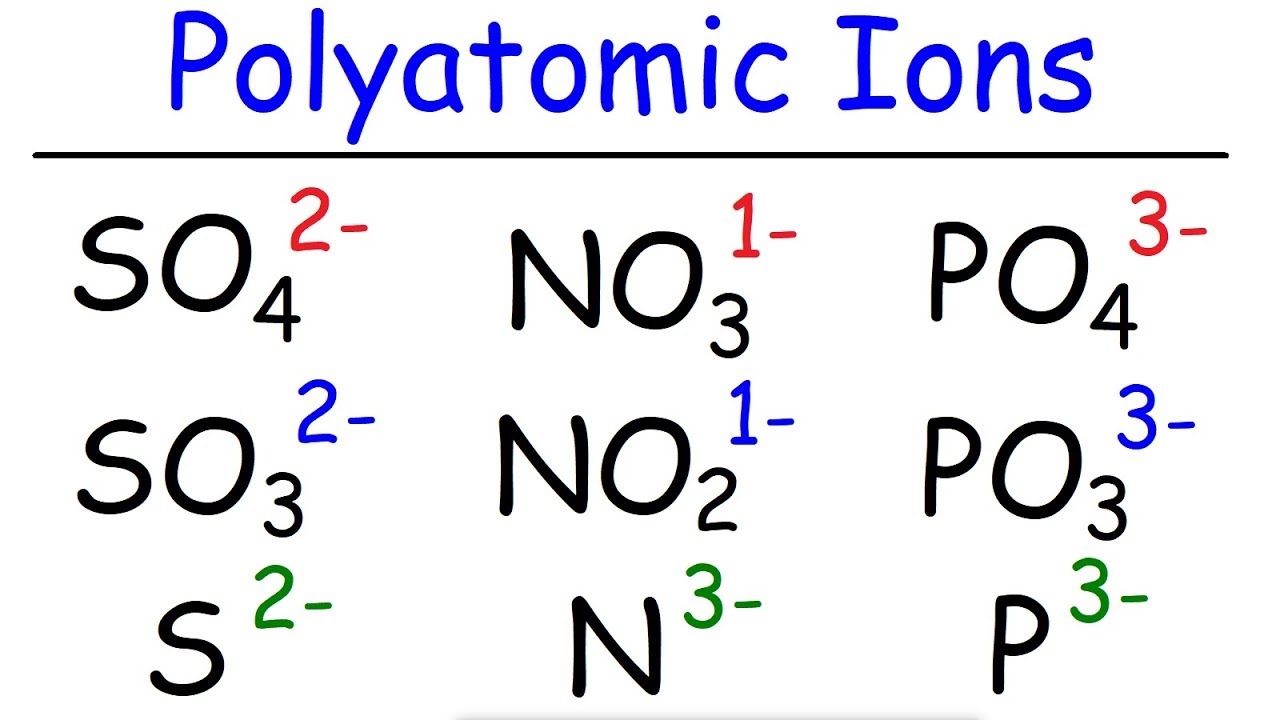
- 🧪 H2SO4 Example: The acid H2SO4 contains the polyatomic ion sulfate, and is named sulfuric acid by following the naming rules.
- 🧪 H2SO3 Example: H2SO3 contains the polyatomic ion sulfite, and is named sulfurous acid by replacing 'ite' with 'ous'.
- 🧪 H2S Example: H2S, with the monoatomic ion sulfide, is named hydrosulfuric acid by adding 'hydro' and 'ic'.
- 🧪 HClO4 Example: HClO4 contains perchlorate ion, and is named perchloric acid by replacing 'ate' with 'ic'.
- 🧪 HCl Example: HCl, with the monoatomic ion chloride, is named hydrochloric acid by adding 'hydro' and replacing 'ide' with 'ic'.
- 🧪 HNO3 and HNO2 Examples: HNO3 is nitric acid with nitrate ion, and HNO2 is nitrous acid with nitrite ion, following the same naming conventions.
- 🧪 HI Example: HI contains the monoatomic ion iodide and is named hydroiodic acid by adding 'hydro' and replacing 'ide' with 'ic'.
- 🧪 HC2H3O2 Example: The polyatomic ion C2H3O2 minus is acetate, and the acid is named acetic acid by keeping the root and replacing 'ate' with 'ic'.
- 🧪 Formula Writing: To write the formula for an acid, identify the polyatomic ion, consider its charge, and add the appropriate number of hydrogens to neutralize it, as shown with phosphoric acid (H3PO4) and phosphous acid (H3PO3).
- 🧪 Carbonic Acid: The formula for carbonic acid is H2CO3, derived from the carbonate ion CO3^2- by adding two hydrogens.
- 🧪 Hydrobromic Acid: The formula for hydrobromic acid is HBr, as it contains the monoatomic ion bromide with a -1 charge, neutralized by one hydrogen.
- 🧪 Hydrocyanic Acid: Despite being a polyatomic ion, HCN follows the naming rule for monoatomic ions with 'hydro' and 'ic', indicating a -1 charge neutralized by one hydrogen.
Q & A
What is the general rule for naming acids that contain polyatomic ions with the word 'a'?
-For acids containing polyatomic ions with the word 'a', you simply add 'ic' to the end of the ion's name and then add the word 'acid'.
How do you name an acid with a polyatomic ion ending in 'i'?
-If the polyatomic ion ends in 'i', you replace it with 's' and add 'acid' at the end.
What prefix should be added to the name of an acid with a monoatomic ion ending in 'ide'?
-For monoatomic ions ending in 'ide', the prefix 'hydro' is added, followed by the suffix 'ic'.
What is the name of the acid H2SO4, and how is it derived from its polyatomic ion?
-H2SO4 is named sulfuric acid. It contains the polyatomic ion sulfate, and by adding 'ic' to 'sulfur', the name becomes sulfuric acid.
How do you name the acid H2SO3, and what polyatomic ion does it contain?
-H2SO3 is named sulfurous acid. It contains the polyatomic ion sulfite, and the 'i' in sulfite is replaced with 'is' to form the name sulfurous acid.
What is the name of the acid H2S, and how is it derived from its monoatomic ion?
-H2S is named hydrosulfuric acid. It contains the monoatomic ion sulfide, and by adding 'hydro' and 'ic' to 'sulfur', the name becomes hydrosulfuric acid.
What is the polyatomic ion in HClO4, and how is the acid named?
-The polyatomic ion in HClO4 is perchlorate. By replacing the 'ate' with 'ic', the acid is named perchloric acid.
How is the acid HClO named, and what polyatomic ion does it contain?
-HClO contains the polyatomic ion hypochlorite. By replacing the 'i' with 'is', the acid is named hypochlorous acid.
What is the name of the acid HCl, and what monoatomic ion does it contain?
-HCl contains the monoatomic ion chloride. By adding the prefix 'hydro' and replacing the 'ide' with 'ic', the acid is named hydrochloric acid.
How do you derive the formula for phosphoric acid from its name?
-Phosphoric acid contains the polyatomic ion phosphate (PO4^3-). To neutralize the -3 charge, three hydrogens are added, resulting in the formula H3PO4.
What is the formula for hydrobromic acid, and how is it derived from its name?
-Hydrobromic acid contains the monoatomic ion bromide (Br^-). To neutralize the -1 charge, one hydrogen is added, resulting in the formula HBr.
How do you write the formula for carbonic acid, and what polyatomic ion is associated with it?
-Carbonic acid contains the polyatomic ion carbonate (CO3^2-). To neutralize the -2 charge, two hydrogens are added, resulting in the formula H2CO3.
What is the formula for hydrocyanic acid, and what polyatomic ion does it contain?
-Hydrocyanic acid contains the polyatomic ion cyanide (CN^-). To neutralize the -1 charge, one hydrogen is added, resulting in the formula HCN.
Outlines
🔬 Naming Acids with Polyatomic Ions
This paragraph introduces the process of naming acids, focusing on the rules for polyatomic ions. It explains how to name sulfuric acid (H2SO4) by identifying the sulfate ion and adding 'ic' to the element sulfur. The paragraph also covers the naming of sulfurous acid (H2SO3) from sulfite, hydrosulfuric acid (H2S) from sulfide, perchloric acid (HClO4) from perchlorate, and hypochlorous acid (HClO) from hypochlorite. The summary provides a step-by-step guide on how to apply specific suffixes and prefixes based on the presence of 'a' or 'i' in the polyatomic ion's name.
🧪 Formula Writing for Acids
This section delves into writing the chemical formulas for acids by working backwards from their names. It discusses the identification of polyatomic ions such as acetate in the case of acetic acid (HC2H3O2) and explains the process of neutralizing the charge of these ions with hydrogen atoms. The paragraph further illustrates how to derive the formulas for phosphoric acid (H3PO4), phosphorous acid (H3PO3), and carbonic acid (H2CO3) by recognizing the associated polyatomic ions and their charges. It also touches on hydrobromic acid (HBr) and hydrocyanic acid (HCN), showing how to determine their formulas based on the presence of monoatomic ions.
📚 Understanding Acid Nomenclature
The final paragraph emphasizes the importance of understanding acid nomenclature, especially for polyatomic ions. It uses hydrocyanic acid as an example to show that despite being a polyatomic ion, it follows the nomenclature rules similar to monoatomic ions. The summary highlights the need to recognize the suffix 'ide' and the prefix 'hydro' to correctly write the formula for such acids, reinforcing the rules introduced in the previous paragraphs.
Mindmap
Keywords
💡Acid
💡Polyatomic Ion
💡Monoatomic Ion
💡Nomenclature
💡Hydro Prefix
💡Suffix
💡Charge
💡Neutralize
💡Binary Acid
💡Oxyacid
💡Formula
Highlights
The video focuses on the rules for naming acids, including polyatomic and monoatomic ions.
For polyatomic ions ending in 'ate', the suffix 'ate' is replaced with 'ic' to form the acid name.
For polyatomic ions ending in 'ite', the suffix 'ite' is replaced with 'ous' to form the acid name.
Monoatomic ions with 'ide' endings require the prefix 'hydro' and the suffix 'ic' for acid naming.
H2SO4 is named sulfuric acid by identifying the sulfate polyatomic ion and applying the naming rules.
H2SO3 is named sulfurous acid by identifying the sulfite polyatomic ion and its 'ite' ending.
H2S is named hydrosulfuric acid by identifying the monoatomic sulfide ion and applying the 'hydro' prefix.
HClO4 is named perchloric acid by identifying the perchlorate polyatomic ion and its 'ate' ending.
HClO is named hypochlorous acid by replacing the 'ite' suffix with 'ous'.
HCl is named hydrochloric acid by identifying the chloride monoatomic ion and applying the 'hydro' prefix.
HNO3 is named nitric acid by identifying the nitrate polyatomic ion and its 'ate' ending.
HNO2 is named nitrous acid by identifying the nitrite polyatomic ion and replacing 'ite' with 'ous'.
HI is named hydroiodic acid by identifying the iodide monoatomic ion and applying the 'hydro' prefix.
HC2H3O2 is named acetic acid by identifying the acetate polyatomic ion and replacing 'ate' with 'ic'.
The formula for phosphoric acid, H3PO4, is derived by neutralizing the phosphate ion with three hydrogens.
The formula for phosphorous acid, H3PO3, is derived by neutralizing the phosphite ion with three hydrogens.
The formula for carbonic acid, H2CO3, is derived by neutralizing the carbonate ion with two hydrogens.
Hydrobromic acid, HBr, is formed by neutralizing the bromide monoatomic ion with one hydrogen.
Hydrocyanic acid, HCN, is formed by neutralizing the cyanide polyatomic ion with one hydrogen.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: