Naming Acids Introduction
TLDRThe video script provides an educational overview on the naming of acids, a crucial aspect of chemistry. It starts with defining an acid as a compound with H+ ions bonded to a negative ion. The script then breaks down common acids into their H+ and negative ion components, emphasizing the importance of recognizing polyatomic ions for accurate naming. The video outlines two main types of acids: those without oxygen and those with oxygen, each having different naming conventions. For non-oxygen acids, the process involves removing the 'ide' from the negative ion's name and inserting it between 'hydro' and 'ic'. Oxygen-containing acids, with polyatomic ions ending in 'ate' or 'ite', follow a different rule set, where 'ate' becomes 'ic' and 'ite' becomes 'ous'. The script also highlights exceptions to these rules, such as phosphoric and sulfuric acids, which do not follow the standard naming convention. To aid memorization, a mnemonic is introduced: 'My ride has hydraulics' for non-oxygen acids, 'I ate something icky' for 'ate' ending ions, and 'Sprite is delicious' for 'ite' ending ions. The video concludes with a teaser for the next video, which will contain more example problems for practice.
Takeaways
- 🔬 An acid is a compound with one or more H+ ions bonded to a negative ion, which can be an individual element or a polyatomic ion.
- 🧬 The name of an acid is based on the name of the negative ion that forms part of the acid, which can be identified by separating the H+ and the negative ion parts.
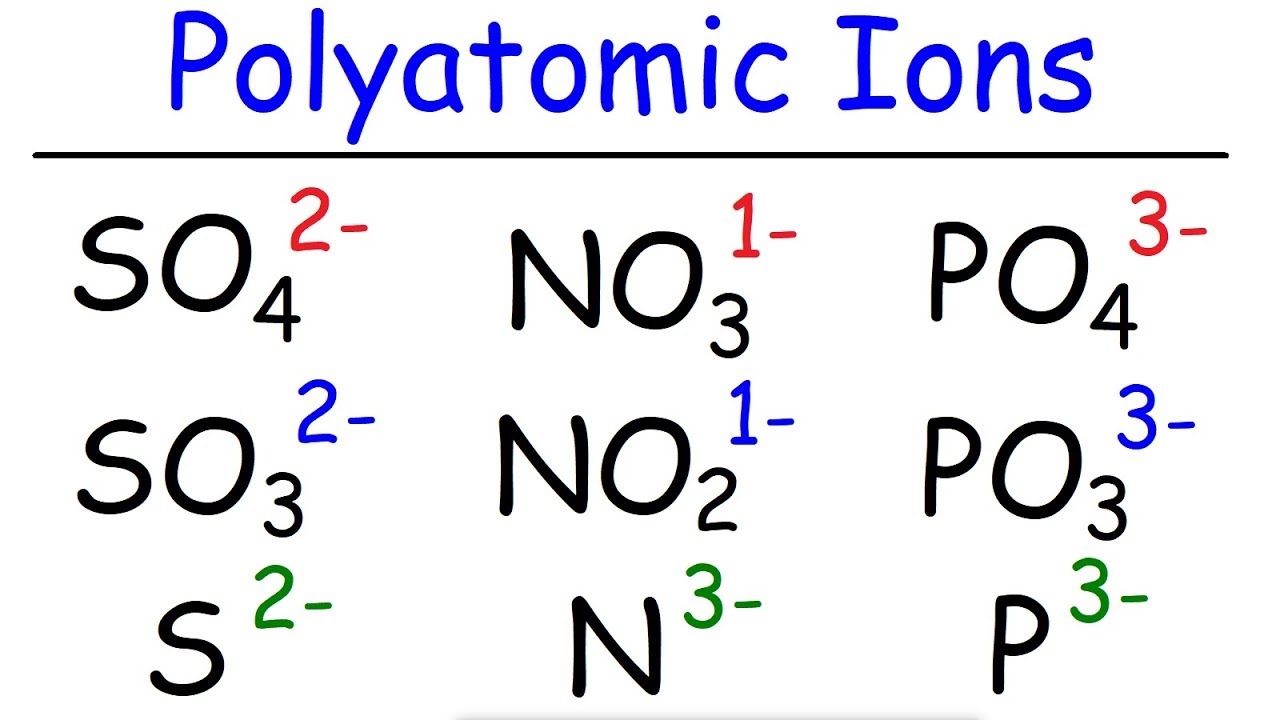
- 📚 Common acids can be broken down into H+ and negative ions, such as F-, Cl-, Br-, or polyatomic ions like NO3- and CO3-2.
- ⚖️ The positive charge from H+ must balance out the negative charge from the negative ion in an acid.
- 📝 For acids without oxygen, if the negative ion ends in 'ide', the 'ide' is removed and 'ic' is added after 'hydro' to form the acid name (e.g., HCl becomes hydrochloric acid).
- 🌐 For acids with oxygen, the negative ions are polyatomic ions that end in 'ate' or 'ite', and their names are used to form the acid name with specific rules.
- 📐 The rule for 'ate' ending ions is to remove the 'ate' and add 'ic' to form the acid name (e.g., nitrate becomes nitric acid).
- 📍 For 'ite' ending ions, the 'ite' is removed and 'ous' is added to form the acid name (e.g., nitrite becomes nitrous acid).
- 🧠 A mnemonic to remember the rules is 'My ride has hydraulics, I ate something icky, Sprite is delicious', which corresponds to the 'ic', 'ate', and 'ous' endings.
- ⚠️ There are exceptions to the naming rules for certain common acids like phosphoric, phosphorous, sulfuric, and sulfurous acids, which do not strictly follow the standard naming convention.
- 📈 Memorizing polyatomic ions and their names is crucial for correctly naming acids containing these ions, especially when they start with 'hypo' or 'per'.
Q & A
What is the basic definition of an acid used in the context of this video?
-An acid is defined as a compound in which one or more H+ ions are bonded or connected to a negative ion.
How are the charges in an ionic acid formula balanced?
-The positive charge from the H+ ions must equal the charge from the negative ion, ensuring the overall charge balance in the acid formula.
What is the basis for naming an acid in chemistry?
-The name of an acid is based on the name of the negative ion that is part of the acid.
How do you name an acid that does not contain oxygen?
-For acids without oxygen, if the negative ion ends in 'ide', you remove the 'ide' and insert the name between 'hydro' and 'ic' to form the acid name.
What is a polyatomic ion?
-A polyatomic ion is a group of elements that together have a charge and are bonded together in a molecule.
How do you name an acid that contains a polyatomic ion ending in 'ate'?
-For polyatomic ions ending in 'ate', you remove the 'ate' and add 'ic' to form the acid name.
What is the rule for naming acids that contain polyatomic ions ending in 'ite'?
-For polyatomic ions ending in 'ite', you remove the 'ite' and add 'ous acid' to form the acid name.
What is the mnemonic provided to remember the rules for naming acids?
-The mnemonic is 'My ride has hydraulics', 'I ate something icky', and 'Sprite is delicious', which helps remember the rules for acids ending in 'ide', 'ate', and 'ite', respectively.
What are the exceptions to the general rules for naming acids?
-There are exceptions for acids containing the negative ions phosphate, phosphite, sulfate, and sulfite, which do not follow the general rules and are named as phosphoric, phosphorous, sulfuric, and sulfurous acid, respectively.
How do you name an acid that contains a polyatomic ion starting with 'hypo' or 'per'?
-For polyatomic ions starting with 'hypo' or 'per', you focus on the ending of the ion. If it ends in 'ate', change it to 'ic', and if it ends in 'ite', change it to 'ous'.
What is the importance of recognizing polyatomic ions when naming acids?
-Recognizing polyatomic ions is crucial as it helps in identifying the correct ending to apply the appropriate naming rule for the acid.
What is the recommendation for someone learning to name acids?
-It is recommended to memorize a list of common polyatomic ions and to watch further example problems in subsequent videos for practice.
Outlines
🌟 Introduction to Acid Nomenclature
This paragraph introduces the topic of acid naming, explaining that an acid is a compound with one or more H+ ions bonded to a negative ion. The video aims to teach viewers how to write names for acids based on their chemical formulas. Common acids are listed, and their composition into H+ and negative ion parts is discussed. The importance of recognizing polyatomic ions and the balance of charges is emphasized. The naming process is outlined, showing that the acid name depends on the negative ion's name. Examples of naming acids without oxygen are provided, using rules that involve removing the 'ide' ending from the negative ion name and inserting it between 'hydro' and 'ic'.
🔍 Naming Acids with Oxygen-Containing Polyatomic Ions
The second paragraph delves into naming acids that include oxygen and polyatomic ions. It stresses the importance of recognizing these ions to correctly name the acids. A chart of polyatomic ions is referenced, and viewers are encouraged to memorize them. The process of naming acids with oxygen-containing ions is explained, highlighting the transformation of the ion's ending from 'ate' to 'ic' for the acid name. Additional examples are given, illustrating the naming process for different polyatomic ions ending in 'ate' and 'ite'. A mnemonic device, 'My ride has hydraulics, I ate something icky, Sprite is delicious,' is introduced to help remember the naming rules.
⚠️ Exceptions to Acid Nomenclature Rules
The third paragraph addresses exceptions to the acid naming rules, particularly for common acids like phosphoric, phosphorous, sulfuric, and sulfurous acids. It clarifies that despite the general rules, these acids have specific names that do not strictly follow the 'ate' to 'ic' or 'ite' to 'ous' conversion. The paragraph also touches on acids containing polyatomic ions starting with 'hypo' or 'per', explaining that the naming process remains the same, focusing only on the ion's ending. The importance of learning these exceptions is emphasized, and viewers are encouraged to watch the next video for more practice with example problems.
Mindmap
Keywords
💡Acid
💡Negative Ion
💡Hydrochloric Acid
💡Polyatomic Ions
💡Nitric Acid
💡Carbonic Acid
💡Nitrous Acid
💡Chromic Acid
💡Pnemonics
💡Phosphoric Acid
💡Hypo and Per Ions
Highlights
An acid is a compound where one or more H+ ions are bonded to a negative ion
Common acids can be broken down into an H+ part and a negative ion part
The name of an acid is based on the name of the negative ion in the acid
Acid names are formed by taking the name of the negative ion and modifying it with 'hydro' and 'ic' or 'ous'
For acids without oxygen, if the negative ion ends in 'ide', remove 'ide' and put 'hydro' and 'ic' before it
For acids with oxygen, the negative ions are polyatomic ions that end in 'ate' or 'ite'
For polyatomic ions ending in 'ate', remove 'ate' and add 'ic' to form the acid name
For polyatomic ions ending in 'ite', remove 'ite' and add 'ous' to form the acid name
Memorizing common polyatomic ions is important for acid naming
A mnemonic to remember acid naming rules is 'My ride has hydraulics' (ID, hydro, ate, ick) and 'I ate something icky' (ate, ic) and 'Sprite is delicious' (ous)
There are a few common exceptions to the acid naming rules, such as phosphoric, sulfuric, and sulfurous acids
For polyatomic ions starting with 'hypo' or 'per', focus on the ending letters to determine the acid name
The video provides a comprehensive introduction to acid naming rules with examples
The next video will provide many example problems for practice with acid naming
Understanding how to separate an acid into its H+ and negative ion components is key to naming it
Recognizing and naming polyatomic ions correctly is crucial for accurately naming acids
The video provides a clear, step-by-step explanation of the rules and exceptions for naming acids
Using the provided mnemonic can greatly simplify and reinforce understanding of acid naming
The video emphasizes the importance of memorizing common polyatomic ions to apply the naming rules effectively
Transcripts
Browse More Related Video

How to Name Acids in Chemistry - [1-2-26]

Naming Acids | How to Pass Chemistry

How To Name Acids - The Fast & Easy Way!
How to Memorize The Polyatomic Ions - Formulas, Charges, Naming - Chemistry

What Is The Bronsted Lowry Theory | Acids, Bases & Alkali's | Chemistry | FuseSchool

How to Speak Chemistrian: Crash Course Chemistry #11
5.0 / 5 (0 votes)
Thanks for rating: