Naming Compounds with Polyatomic Ions
TLDRIn this educational video, Melissa Maribel explains the process of naming ionic compounds with polyatomic ions. She emphasizes three key points: naming conventions, balancing charges, and memorizing common polyatomic ions. Through three examples, she demonstrates how to derive chemical formulas from compound names and vice versa. Maribel also shares a memory trick for polyatomic ions and offers additional resources for mastering the topic.
Takeaways
- 📘 Naming ionic compounds with polyatomic ions involves the metal name followed by the polyatomic ion name, all in lowercase.
- 🔍 When converting from compound name to chemical formula, it's crucial to balance the charges of the ions.
- 📝 Memorizing the charges of common polyatomic ions is essential for correctly naming compounds.
- 🧩 Polyatomic ions are composed of two or more atoms with an overall charge.
- 🌰 Example 1: Magnesium carbonate has balanced charges (Mg^2+ and CO3^2-), resulting in the formula MgCO3.
- 🌰 Example 2: Aluminum sulfate requires balancing charges, resulting in the formula Al2(SO4)3.
- 🌰 Example 3: Ammonium sulfate involves both polyatomic ions, leading to the formula (NH4)2SO4.
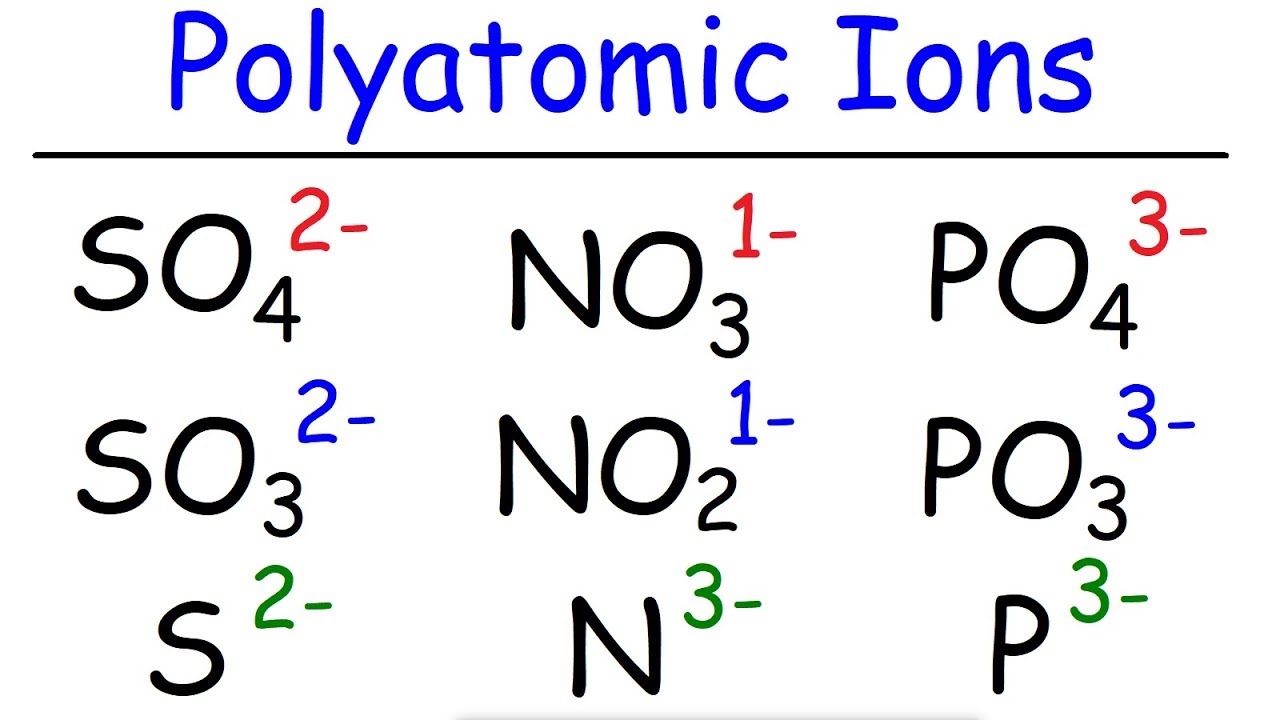
- 📚 A list of common polyatomic ions should be provided by the teacher or found in educational materials.
- 📈 To convert from chemical formula to compound name, identify the metal and polyatomic ion names.
- 📝 A mnemonic trick for memorizing polyatomic ions: 'ates' have one more oxygen atom than 'ites'.
- 🛍️ Additional resources, including a how-to guide on naming compounds and acids, are available for further learning.
Q & A
What is the main purpose of the video script?
-The main purpose of the video script is to teach viewers how to name compounds with polyatomic ions, providing examples and tips for understanding and memorizing the process.
Who is the presenter of the video script?
-The presenter of the video script is Melissa Maribel, who introduces herself as the personal tutor.
What are the three important things to know for naming compounds with polyatomic ions according to the script?
-The three important things to know are: 1) The name of an ionic compound with a polyatomic ion consists of the metal name followed by the polyatomic ion name in lowercase. 2) When finding the chemical formula from the compound name, charges must be balanced. 3) Polyatomic ions are composed of two or more atoms with an overall charge.
Why is it necessary to memorize the charges of common polyatomic ions?
-Memorizing the charges of common polyatomic ions is necessary to correctly balance the charges when finding the chemical formula from the compound name for ionic compounds.
What is the chemical formula for magnesium carbonate as given in the script?
-The chemical formula for magnesium carbonate is MgCO3, as the charges of magnesium (2+) and carbonate (2-) are already balanced.
How does the script suggest balancing charges in ionic compounds?
-The script suggests that to balance charges in ionic compounds, the charge of the polyatomic ion becomes the subscript for the metal, and vice versa.
What is the trick provided in the script to memorize polyatomic ions faster?
-The trick provided is that most polyatomic ions end in 'ite' or 'ate', with 'ates' having one more oxygen atom than 'ites'. Memorizing the 'ates' can help reduce the memorization effort by half.
What is the chemical formula for aluminum sulfate as derived in the script?
-The chemical formula for aluminum sulfate is Al2(SO4)3, reflecting the balance of aluminum's 3+ charge with sulfate's 2- charge.
How does the script explain the naming of the compound from its chemical formula?
-The script explains that to name a compound from its chemical formula, one should identify the name of the metal and the name of the polyatomic ion, and then combine them accordingly.
What is the compound name for the chemical formula (NH4)2SO4 as per the script?
-The compound name for the chemical formula (NH4)2SO4 is ammonium sulfate, identifying NH4 as ammonium and SO4 as sulfate.
What additional resources does Melissa Maribel offer to help with understanding compound naming?
-Melissa Maribel offers a how-to guide on naming compounds and acids, which includes detailed examples and explanations, as well as other resources like homework help and tutoring.
Outlines
🧪 Chemistry Compound Naming Basics
In this segment, Melissa Maribel introduces the fundamentals of naming compounds with polyatomic ions. She emphasizes three key points: the naming convention where the metal name precedes the polyatomic ion in lowercase, the necessity to balance charges when deriving chemical formulas, and the importance of memorizing common polyatomic ions. She also provides a list of these ions and offers a helpful mnemonic for memorization, noting that most polyatomic ions end in 'ite' or 'ate', with 'ate' ions having an extra oxygen atom. The paragraph concludes with three examples illustrating the process of converting between compound names and chemical formulas, highlighting the importance of charge balance and the use of parentheses for polyatomic ions requiring subscripts.
📚 Additional Resources for Learning Chemistry
The second paragraph focuses on the availability of supplementary educational materials designed to aid in the understanding of chemistry concepts, particularly compound naming. Melissa Maribel mentions a how-to guide she created, which includes over 30 pages of detailed examples and explanations. She also invites viewers to access additional resources such as homework help and tutoring services, all of which are available through the provided link in the description box. The paragraph ends on an encouraging note, urging learners to stay determined in their educational journey.
Mindmap
Keywords
💡Compound Name
💡Chemical Formula
💡Polyatomic Ion
💡Ionic Compound
💡Charge Balancing
💡Subscript
💡Metal
💡Memorization
💡Acetate
💡Hydroxide
💡Ammonium
💡Nitrate
Highlights
Introduction to the process of naming compounds with polyatomic ions by Melissa Maribel.
Three essential things to know for naming compounds with polyatomic ions.
The naming of ionic compounds with polyatomic ions should consist of the metal name followed by the ion name, all in lowercase.
Balancing out the charges is necessary when finding the chemical formula from the compound name.
The importance of memorizing the charges of common polyatomic ions.
Definition of polyatomic ions as ions composed of two or more atoms with an overall charge.
Example 1: Finding the chemical formula for magnesium carbonate with same charges.
Explanation of how to identify the symbol and charge for magnesium and carbonate.
Example 2: Balancing charges in aluminum sulfate to find the chemical formula.
How to adjust subscripts to balance charges in ionic compounds.
Example 3: Finding the chemical formula for ammonium sulfate with both polyatomic ions.
Method to identify and balance charges of each polyatomic ion in a compound.
Conversion from chemical formula to compound name for ammonium nitrate.
Trick to memorize polyatomic ions: Most end in 'ite' or 'ate', with 'ate' having one more oxygen.
Introduction of Melissa Maribel's how-to guide for naming compounds and acids.
Offering of additional resources such as homework help and tutoring.
Encouragement to stay determined in learning the process of naming compounds.
Transcripts
Browse More Related Video

How to Predict Products of Chemical Reactions | How to Pass Chemistry

Writing Formulas with Polyatomic Ions

Balancing Chemical Equations with Polyatomic Ions

How To Name Ionic Compounds With Transition Metals
How to Memorize The Polyatomic Ions - Formulas, Charges, Naming - Chemistry

Naming Ionic and Molecular Compounds | How to Pass Chemistry
5.0 / 5 (0 votes)
Thanks for rating: