How to Name Acids in Chemistry - [1-2-26]
TLDRThis chemistry lesson introduces the concept of acids, explaining that they are substances which yield hydrogen ions (protons) when dissolved in water. The script delves into everyday examples of acids, such as lemon juice and vinegar, and touches on the reactivity of acids, which can 'eat through' materials due to their chemical properties. It outlines a basic definition of an acid and emphasizes the importance of understanding this definition for naming acids correctly. The lesson then focuses on the naming conventions for acids, categorizing them into three main groups based on the type of anion they contain: ide, ate, and ite ions. Through examples, it demonstrates how to name acids by identifying the anion and applying specific suffix rules. The script also previews further lessons that will explore the details of acids and their reactivity.
Takeaways
- 🍋 An acid is a substance that has a sharp taste and can be reactive, often found in everyday items like lemons and vinegar.
- 🌡 The chemical definition of an acid is a substance that yields hydrogen ions (protons) when dissolved in water.
- 🔬 Hydrochloric acid, a common stomach acid, is an example of an acid that can be reactive and is important in the study of chemistry.
- 🔀 Acids can cause chemical reactions with other substances, which is why they can 'eat through' or react with materials like metals.
- 📚 The strength of an acid is related to the concentration of hydrogen ions in the solution; the more hydrogen ions, the stronger the acid.
- 🧪 Hydrogen ions come from the acid when it is dissolved in water, leading to a solution with free protons that can react with other substances.
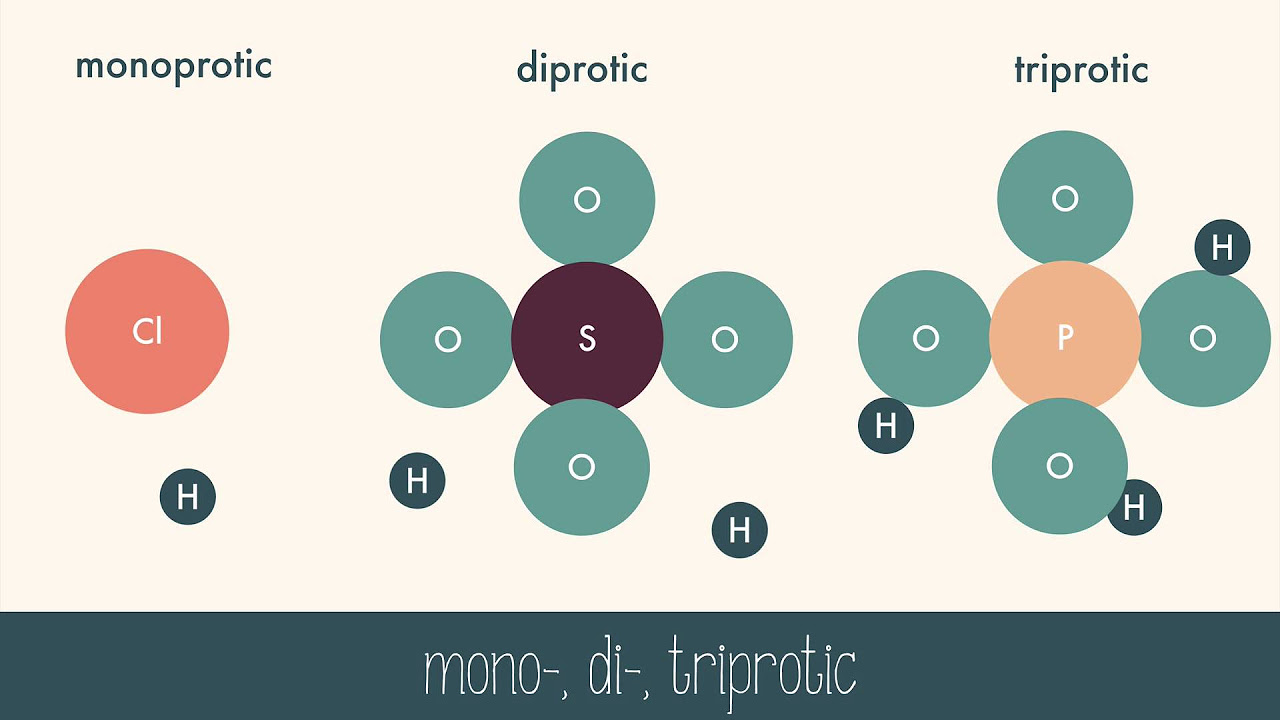
- 📐 Naming acids involves identifying the anion (negative ion) and following specific conventions based on the type of anion, such as 'hydro' for ide ions and 'ic' or 'ous' suffixes for ate and ite ions.
- 📚 When the anion is a simple ide ion (like chloride), acids are named as 'hydro' followed by the element name changed to 'ic' and 'acid'.
- 🧲 For polyatomic anions with ate endings (like sulfate), acids are named directly based on the anion, changing 'ate' to 'ic' without 'hydro'.
- ⚗️ If the anion ends in ite, the acid is named by changing 'ite' to 'ous' and not starting with 'hydro', as seen in chlorite becoming chlorous acid.
- 🔑 Understanding the naming conventions and the types of anions helps in identifying and naming acids correctly, which is crucial for solving chemistry problems.
Q & A
What is the chemical definition of an acid?
-An acid is a substance that yields hydrogen ions (protons) when dissolved in water.
What are some everyday examples of acids?
-Examples include lemon juice, vinegar, and stomach acid (hydrochloric acid).
Why do acids have a sharp or pungent taste?
-The sharp taste is due to the acidic nature of these substances, which can be felt when they come into contact with the taste buds.
What is the basic definition of an acid for the purpose of this lesson?
-For this lesson, an acid is defined as a substance that yields hydrogen ions when dissolved in water.
How are acids named in general?
-Acids are generally named based on their anion (negative ion), with specific rules for naming depending on the type of anion involved.
What is the naming convention for acids with an anion that ends in 'ide'?
-For acids with an anion ending in 'ide', the 'ide' is changed to 'ic' and 'hydro' is added in front of it, followed by the word 'acid'.
Can you provide an example of an acid with an 'ate' anion and how it is named?
-An example is sulfuric acid (H2SO4), where the anion is sulfate. It is named based on the anion without the 'hydro' prefix, resulting in 'sulfuric acid'.
What is the process for naming acids with an 'ite' anion?
-For acids with an 'ite' anion, the 'ite' suffix is changed to 'ous', and the acid is named based on the resulting combination without the 'hydro' prefix.
How does the strength of an acid relate to the number of hydrogen ions present?
-The strength of an acid is related to the concentration of hydrogen ions present; the more hydrogen ions, the stronger the acid.
What is the pH scale and how does it measure the strength of an acid?
-The pH scale measures the strength of an acid by quantifying the concentration of hydrogen ions in a solution; a lower pH indicates a higher concentration of hydrogen ions and thus a stronger acid.
How can you determine if a compound is an acid?
-A compound is an acid if it contains hydrogen that can lose an electron (form hydrogen ions) when dissolved in water.
Can you explain the 'crisscross' method mentioned in the script for determining the formula of an acid?
-The 'crisscross' method involves determining the charges of the ions in the acid and then using those charges to balance the formula. For example, if you know the acid name or anion, you can identify the charge on the anion and use the crisscross method to find the correct subscripts for the hydrogen ions to balance the overall charge to zero.
Outlines
🧪 Introduction to Acids and Their Naming
This paragraph introduces the topic of acids, explaining their everyday presence and the need to understand their chemical nature. Acids are defined as substances that yield hydrogen ions (protons) when dissolved in water. Common examples like lemon and vinegar are given to illustrate the concept. The paragraph also touches on the reactivity of acids and sets the stage for a detailed exploration of acids in future lessons. The importance of the definition of an acid is emphasized, and the basics of acid naming are introduced, with hydrogen typically listed first in their chemical formulas.
🌡 Understanding Acid Reactivity and Naming Conventions
This section delves into the reactivity of acids, explaining that strong acids can react vigorously with other substances due to their high concentration of hydrogen ions. The concept of acid strength and the pH scale are briefly introduced, with the promise of a more in-depth discussion later. The paragraph then focuses on the naming of acids, starting with the general rule that acids are named based on their anions. Examples such as hydrochloric acid (HCl) are used to illustrate the naming process, where hydrogen is always written first, followed by the anion.
🔬 Naming Acids Based on Anion Types
The paragraph explains the process of naming acids according to the type of anion they contain. There are three main categories: acids with an ide anion (e.g., chloride, bromide) are named as 'hydro某ick acid', those with an ate anion (e.g., sulfate, nitrate) are named directly from the anion without the 'hydro' prefix, and those with an ite anion (e.g., chlorite) are named by changing the suffix to 'ous'. Examples such as sulfuric acid (H2SO4) and chloric acid (HClO2) are provided to demonstrate these naming conventions.
📚 Applying Acid Naming Rules to Common Examples
This section applies the previously discussed rules to name various acids. It covers how to identify the anion in an acid and how to use the suffixes 'ide', 'ate', and 'ite' to determine the acid's name. Examples include hydrobromic acid (HBr), bromic acid (HBrO3), phosphoric acid (H3PO4), and others, with explanations on how to match the anion to the correct naming convention.
🔍 Reverse Engineering Acid Names to Formulas
The paragraph demonstrates how to reverse engineer the names of acids to their chemical formulas. It explains the process of identifying the anion from the acid name and using the crisscross method to determine the correct formula. Examples such as hypochlorous acid (HClO), iodate acid (HIO3), and sulfurous acid (H2SO3) are used to illustrate this process, showing how to translate from the name to the formula and vice versa.
🔬 Conclusion on Acids: Definitions, Reactivity, and Naming
In conclusion, the paragraph summarizes the key points about acids: their definition as substances that yield hydrogen ions in water, their reactivity based on the concentration of these ions, and the naming conventions based on the anion type. It emphasizes the importance of understanding these concepts for future studies in chemistry and encourages practice with the provided examples to solidify the knowledge.
Mindmap
Keywords
💡Acid
💡Hydrogen Ion
💡Anion
💡Hydrochloric Acid
💡Polyatomic Ion
💡Naming Conventions
💡Reactivity
💡pH Scale
💡Ionic Compounds
💡Criscross Method
Highlights
Introduction to the concept of acids and the process of naming them.
Everyday experience with acids like lemon and vinegar, and the chemical definition of an acid.
Explanation of acids' reactivity and their ability to 'eat through' substances due to chemical reactions.
Basic definition of an acid as a substance that yields hydrogen ions when dissolved in water.
Importance of understanding the definition of acids for further learning and naming.
Naming acids by the anion present, with examples of hydrochloric acid and its naming convention.
Differentiation between acids with simple anions and those with polyatomic ions.
How to name acids with polyatomic anions like sulfate and chlorite.
The process of identifying the anion to determine the acid's name and strength.
Explanation of the crisscross method to predict subscripts in acid formulas.
Reversing the naming process to determine the chemical formula from the acid name.
Practice examples for naming acids with different anions like bromate and phosphate.
Understanding the exceptions and slight variations in naming conventions for certain acids.
The relationship between the number of hydrogen ions and the strength of an acid.
Introduction to the pH scale as a measure of acidity and its relation to hydrogen ion concentration.
Final summary of the lesson, emphasizing the importance of knowing how to name acids and their properties.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: