[H2 Chemistry] 2023 Topic 2 Chemical Bonding 4
TLDRThis chemistry lecture delves into chemical bonding, focusing on intermolecular forces, hydrogen bonding, and the physical properties of different lattice structures. It explains how these forces influence solubility, melting points, and electrical conductivity across elements. The lecture also clarifies misconceptions about the strength of various bonds and emphasizes the importance of understanding chemical bonding for further studies in chemistry.
Takeaways
- 🔬 Chemical bonding is a complex topic that deepens with further education in chemistry, and complete understanding is not expected early on.
- 🧲 Intermolecular forces of attraction, including hydrogen bonding, are electrostatic in nature and play a crucial role in the physical properties of substances.
- 🌐 Hydrogen bonding is a special type of strong intermolecular force that occurs when hydrogen is bonded to highly electronegative elements like fluorine, oxygen, or nitrogen.
- 💧 Water's high boiling point is due to its extensive hydrogen bonding, which requires more energy to overcome compared to other diatomic molecules.
- 🌡 The strength of dispersion forces depends on the size and polarizability of the electron cloud, affecting the melting and boiling points of molecules.
- 🌟 Hydrogen bonds are not only strong but also extensive in water, contributing to its high surface tension and unique properties that support life.
- 🏔️ Diamond has a high melting point due to its strong covalent lattice structure, where each carbon atom is bonded to four others in a tetrahedral arrangement.
- 📜 Graphite, another allotrope of carbon, has a layered structure with strong covalent bonds within layers and weak dispersion forces between them, making it soft and slippery.
- 🌌 The melting points of substances can be explained by the types of bonds and structures they possess, from metallic to ionic to covalent networks.
- 🧴 Solubility of substances in solvents like water or organic solvents depends on the ability of solute-solvent interactions to overcome the existing intermolecular or intramolecular forces within the solute and solvent.
Q & A
What are intermolecular forces of attraction?
-Intermolecular forces of attraction are forces that occur between molecules, influencing the physical properties of substances. They include hydrogen bonding, dipole-dipole interactions, and London dispersion forces.
What is the difference between permanent dipole and induced dipole interactions?
-Permanent dipole interactions occur between molecules that have a permanent separation of charge, whereas induced dipole interactions occur when a temporary dipole in one molecule induces a dipole in a nearby molecule.
What is hydrogen bonding and why is it considered a special category of intermolecular forces?
-Hydrogen bonding is a strong type of dipole-dipole interaction that occurs between a hydrogen atom bonded to a highly electronegative atom (fluorine, oxygen, or nitrogen) and a lone pair of electrons on another electronegative atom. It is considered special due to its significant strength compared to other dipole interactions.
Why do molecules like trichloromethane (CHCl3) exhibit permanent dipole interactions?
-Trichloromethane exhibits permanent dipole interactions because the molecule has a permanent dipole moment due to the difference in electronegativity between hydrogen and chlorine atoms.
Why are larger electron clouds more easily distorted, leading to stronger induced dipole interactions?
-Larger electron clouds are more easily distorted because they are less tightly held by the nucleus, making it easier for temporary dipoles to form, which leads to stronger induced dipole interactions.
How do the boiling points of substances relate to the strength of intermolecular forces?
-The boiling points of substances are higher when the intermolecular forces are stronger because more energy is required to overcome these forces and transition the substance from liquid to gas.
What role do relative molecular mass and electron cloud size play in determining the strength of London dispersion forces?
-The relative molecular mass and the size of the electron cloud determine the polarizability of a molecule. Larger molecules with more electrons have greater dispersion forces because their electron clouds can be more easily distorted, leading to stronger instantaneous dipole-induced dipole interactions.
Why do substances with hydrogen bonding have higher boiling points compared to those with only dipole-dipole interactions?
-Substances with hydrogen bonding have higher boiling points because hydrogen bonds are significantly stronger than regular dipole-dipole interactions, requiring more energy to break these bonds during the phase change from liquid to gas.
What is the importance of studying hydrogen bonds in biological systems?
-Hydrogen bonds are crucial in biological systems as they play key roles in the structure and function of biomolecules like DNA, where they help stabilize the double helix, and in proteins, where they contribute to secondary and tertiary structures.
Why is water's boiling point much higher than expected based on its molecular weight?
-Water's boiling point is much higher than expected based on its molecular weight due to the extensive hydrogen bonding between water molecules, which requires significant energy to overcome.
Outlines
📘 Introduction to Chemical Bonding Lecture
The lecturer begins by welcoming students to the final lecture on chemical bonding, emphasizing the importance of revisiting previous lectures for better understanding. The focus of this lecture is on section 9, which covers intermolecular forces of attraction. Concepts such as permanent dipoles, induced dipoles, and the categorization of intermolecular forces are introduced. Examples include trichloromethane, dibromine, and hydrogen bonding.
📘 Detailed Explanation of Hydrogen Bonding
The lecturer delves into the specifics of hydrogen bonding, explaining its origin and significance. Examples involving hydrogen bonds with fluorine, nitrogen, and oxygen are discussed, illustrating the strong electrostatic interactions. The concept of protonic hydrogens and their role in hydrogen bonding is introduced, along with a comparison of the strength of hydrogen bonds relative to other intermolecular forces.
📘 Factors Influencing Intermolecular Forces
The lecturer compares different types of intermolecular forces, explaining that the size and polarizability of electron clouds affect their strength. Examples include sulfur (S8) and water (H2O), highlighting how larger electron clouds lead to stronger instantaneous dipole-induced dipole interactions. A summary table is provided to determine the types of intermolecular forces present in various molecules.
📘 Practical Applications of Dispersion Forces
The discussion includes real-world examples of dispersion forces, such as geckos climbing walls and the functionality of Velcro. The lecturer links a video for further understanding. An exercise follows, asking students to explain the volatility trend of group 17 elements (fluorine, chlorine, bromine, iodine) based on their intermolecular forces.
📘 Volatility Trends in Group 17 Elements
The lecturer explains the trend in volatility among group 17 elements, focusing on the size and polarizability of their electron clouds. Fluorine and chlorine, being gases, are the most volatile, while iodine, a solid, is the least. The strength of intermolecular forces increases from fluorine to iodine, correlating with the elements' physical states at room temperature.
📘 Boiling Points and Intermolecular Forces
The lecturer discusses the relationship between molecular size, electron cloud distortion, and boiling points. Examples of pentane and 2,2-dimethylpropane illustrate how elongated electron clouds with larger surface areas result in stronger dispersion forces and higher boiling points. The significance of dispersion forces is emphasized, even in simple molecular interactions.
📘 Hydrogen Bonding in Different Molecules
Hydrogen bonding's impact on molecular properties is examined through examples like water (H2O), ammonia (NH3), and hydrogen fluoride (HF). The strength and extensiveness of hydrogen bonds are compared, with water forming the most extensive hydrogen bonds. Misconceptions about hydrogen bonds being covalent bonds are clarified.
📘 Intramolecular vs. Intermolecular Hydrogen Bonds
The difference between intermolecular and intramolecular hydrogen bonds is explained using examples like 2-nitrophenol and 4-nitrophenol. Intramolecular hydrogen bonds form within a single molecule, leading to less extensive intermolecular bonding. The stability and formation of hydrogen bonds in cyclic structures are highlighted.
📘 Aromatic Stability and Benzene Structure
The lecturer introduces the concept of aromatic stability, using benzene as an example. The alternating double and single bonds create a delocalized pi electron cloud, contributing to benzene's stability. The skeletal structure of benzene and its representation in chemical diagrams are explained.
📘 Unique Properties of Ice and Water
The lecturer discusses the unique properties of ice and water due to hydrogen bonding. Ice's open lattice structure makes it less dense than water, allowing it to float and enabling marine life to survive in winter. Water's high surface tension, caused by hydrogen bonding, is also mentioned.
📘 Covalent Lattices and Their Properties
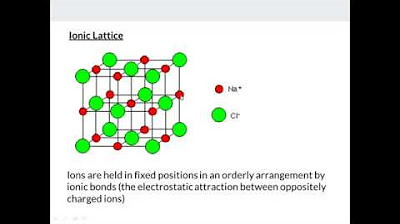
Covalent lattices, including diamond and graphite, are described. Diamond's tetrahedral structure and graphite's layered structure with delocalized electrons are highlighted. The properties of these structures, such as hardness, electrical conductivity, and solubility, are explained in detail.
📘 Summary of Chemical Bonding Concepts
The lecturer summarizes the key concepts of chemical bonding covered in the lecture series. Emphasis is placed on understanding the types of interactions within and between molecules, as well as the impact of these interactions on physical properties like melting and boiling points, solubility, and electrical conductivity.
Mindmap
Keywords
💡Chemical Bonding
💡Intermolecular Forces
💡Electronegativity
💡Polar Molecules
💡Hydrogen Bonding
💡Giant Covalent Lattice
💡Metallic Bonding
💡Dispersion Forces
💡Solubility
💡Ionic Lattice
💡Electron Cloud
💡Hybridization
Highlights
Chemical bonding is a complex topic that deepens with further education, emphasizing the importance of revisiting previous lectures for better understanding.
Intermolecular forces are categorized and explained, including permanent and induced dipoles, highlighting their electrostatic nature similar to intramolecular forces.
Trichloromethane is used as an example to illustrate the concept of permanent dipole interactions between molecules.
Dibromine and molecular bromine are discussed to explain instantaneous dipole-induced dipole interactions, emphasizing the role of electron cloud distortion.
Hydrogen bonding is introduced as a special category of dipole-dipole interaction, with a focus on its strength and significance in chemistry.
The concept of protonic hydrogens is explained, relating to their ability to form hydrogen bonds with electronegative elements like fluorine, oxygen, and nitrogen.
A comparison of intermolecular forces is made, correcting the misconception that hydrogen bonding is always stronger than other interactions.
The size of the electron cloud and its relation to the strength of dispersion forces are discussed, using S8 and H2O as examples.
A flowchart is introduced to determine the types of intermolecular forces, providing a systematic approach to understanding these attractions.
London dispersion forces are explained as a fundamental intermolecular force present between all molecules, including their dependence on molecular size and polarizability.
The importance of dispersion forces in everyday life is highlighted, with examples such as gecko feet adhesion and Velcro.
The volatility of Group 17 elements is discussed in relation to their molecular structure and intermolecular forces, illustrating the concept with fluorine, chlorine, bromine, and iodine.
The solubility of different molecules in water is explained based on their ability to form hydrogen bonds and the strength of their intermolecular forces.
The strength and extent of hydrogen bonding are compared between HF, H2O, and ammonia, showing how they affect the boiling points of these molecules.
The phenomenon of ice being less dense than water due to its open lattice structure is discussed, along with its implications for marine life.
The high surface tension of liquid water is mentioned, explaining phenomena such as objects floating on water and the ability of certain insects to 'skate' on water surfaces.
Covalent lattices and their physical properties are introduced, differentiating between simple molecular and giant covalent structures.
The melting and boiling points of simple molecular compounds are discussed in relation to the strength of their intermolecular forces, using argon and HCL as examples.
The electrical conductivity of different substances is related to their structure and bonding, with metals like sodium and aluminum being good conductors and simple molecular compounds being non-conductors.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: