1.6 Intermolecular Forces | Organic Chemistry
TLDRThis video script delves into the intricacies of intermolecular forces, a pivotal topic in organic chemistry. It outlines the various types of these forces, including hydrogen bonding, dipole-dipole interactions, London dispersion forces, and briefly mentions ion-dipole forces. The script emphasizes that intermolecular forces, while weaker than chemical bonds, play a crucial role in determining the physical properties of substances, notably their boiling and melting points, viscosity, and surface tension. The principle of 'like dissolves like' is also discussed, highlighting the solubility of polar and non-polar substances in respective media. The video is educational, providing a comprehensive review suitable for students of organic chemistry, and is complemented by examples and comparisons to enhance understanding.
Takeaways
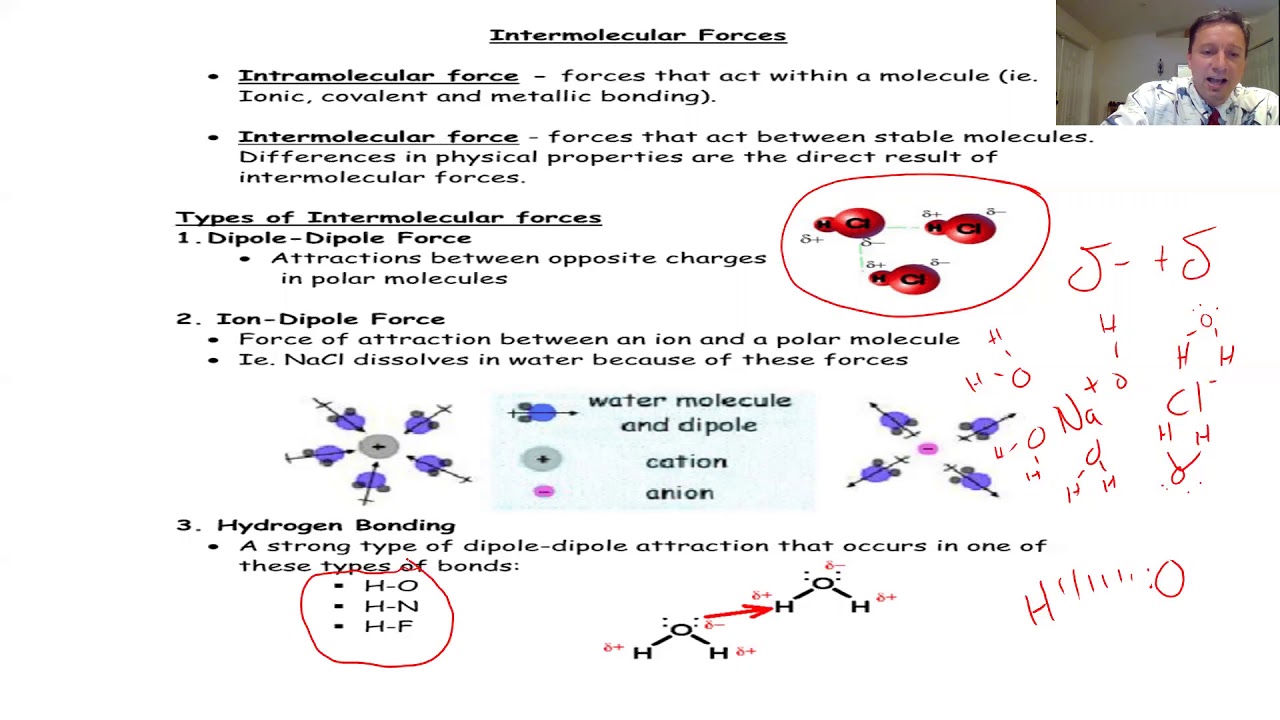
- 🔬 **Intermolecular Forces Overview**: The lesson covers hydrogen bonding, dipole-dipole forces, London dispersion forces, and briefly mentions ion-dipole forces, focusing on their role in molecular compounds.
- ⚛️ **Polarity and Forces**: Polarity, discussed in the previous lesson, sets the stage for understanding dipole-dipole forces, as polar molecules exhibit these forces due to the separation of charges.
- 💧 **Hydrogen Bonding**: Described as a strong dipole-dipole force, hydrogen bonding occurs when hydrogen is bonded to highly electronegative elements (F, O, or N), and it's significantly weaker than a covalent bond.
- 🔆 **Dipole-Dipole Interactions**: These forces are present between polar molecules, with the strength of the force increasing with the molecule's polarity.
- 🤝 **London Dispersion Forces**: Considered the weakest of the intermolecular forces, these are temporary dipoles that occur in all molecules, even nonpolar ones, and are influenced by size, surface area, and polarizability.
- ⚡ **Ion-Dipole Forces**: These forces are stronger than the other three but are only present in mixtures, such as when ionic compounds are dissolved in polar liquids.
- 🌡️ **Boiling Points and Forces**: The boiling point of a substance is directly related to its intermolecular forces; stronger forces lead to higher boiling points.
- 🧊 **Melting Points and Branching**: While boiling points generally follow a consistent trend with intermolecular forces, melting points can be affected by branching and symmetry, leading to exceptions in the expected trend.
- 🌟 **Size and Surface Area**: The size and surface area of molecules, particularly in nonpolar compounds, play a significant role in the strength of London dispersion forces and thus impact boiling points.
- 🍃 **Branching in Hydrocarbons**: More branched hydrocarbons have a lower surface area, which can lead to lower boiling points despite having the same molecular weight as their unbranched counterparts.
- ➿ **Solubility Principle**: The 'like dissolves like' principle states that polar solutes dissolve in polar solvents, and non-polar solutes dissolve in non-polar solvents, highlighting the importance of molecular polarity in solubility.
Q & A
What are the three main types of intermolecular forces discussed in the lesson?
-The three main types of intermolecular forces discussed are hydrogen bonding, dipole-dipole forces, and London dispersion forces.
Why are intermolecular forces weaker than ionic and covalent bonds?
-Intermolecular forces are weaker because they involve interactions between separate molecules rather than the strong chemical bonds that hold atoms together within a molecule.
What is the strongest intermolecular force and how does it compare to a covalent bond?
-Hydrogen bonding is the strongest intermolecular force. However, it is significantly weaker than a covalent bond, usually around 25 to 100 times weaker.
What is a requirement for a molecule to participate in hydrogen bonding?
-For a molecule to participate in hydrogen bonding, it must contain a hydrogen atom bonded to a fluorine, oxygen, or nitrogen atom.
How does the size of a molecule affect its intermolecular forces?
-The size of a molecule affects its intermolecular forces because larger molecules have more surface area, which can lead to stronger London dispersion forces.
What is the principle that governs solubility of compounds?
-The principle governing solubility is 'like dissolves like,' meaning polar solutes dissolve in polar liquids and non-polar solutes dissolve in non-polar liquids.
Why does branching in hydrocarbons typically lead to lower boiling points?
-Branching in hydrocarbons leads to more compact structures with lower surface areas, which results in weaker London dispersion forces and thus lower boiling points.
How does the presence of hydrogen bonding affect the boiling point of a molecule?
-The presence of hydrogen bonding significantly increases the boiling point of a molecule due to the strong intermolecular forces involved in hydrogen bonding.
What is the role of polarizability in London dispersion forces?
-Polarizability affects London dispersion forces by determining how easily an electron cloud can be distorted to create temporary dipoles, which influences the strength of the dispersion forces.
Why might a non-polar molecule have a higher boiling point than a polar molecule of similar size?
-A non-polar molecule might have a higher boiling point than a polar molecule of similar size if the non-polar molecule is significantly larger, compensating for the lack of dipole-dipole forces with stronger London dispersion forces.
How does the structure of a molecule, specifically branching, affect its melting point?
-Branching can affect a molecule's melting point by altering how the molecule packs into a crystal. Highly branched and symmetrical structures may pack better, leading to higher melting points, but this trend is not as consistent as with boiling points.
Outlines
🔬 Introduction to Intermolecular Forces
The video begins by introducing intermolecular forces as the topic of the last lesson in the first chapter of the organic chemistry playlist. It covers hydrogen bonding, dipole-dipole forces, London dispersion forces, and briefly mentions ion-dipole forces. The lesson is a continuation from a discussion on polarity, which is essential for understanding dipole-dipole forces. The video emphasizes that intermolecular forces are weaker than chemical bonds and only apply to molecular compounds, not ionic or network covalent compounds. It also explains that these forces are responsible for the 'stickiness' between separate molecules and are influenced by the presence of positive and negative charges.
🗜️ Strength and Types of Intermolecular Forces
The paragraph explains the hierarchy of intermolecular forces starting with hydrogen bonding as the strongest, followed by dipole-dipole forces, and then London dispersion forces. It also notes that ion-dipole forces can be stronger but are only present in mixtures. The video uses water as an example of a molecule capable of hydrogen bonding due to its polar nature. The requirements for hydrogen bonding are discussed, specifying that it involves hydrogens bonded to fluorine, oxygen, or nitrogen. The difference between hydrogen bond donors and acceptors is clarified using water and formaldehyde as examples. The paragraph concludes by discussing the temporary and weak nature of London dispersion forces, which are present in all molecules but are the weakest of the three forces discussed.
🧲 Factors Affecting London Dispersion Forces
This section delves into the factors that affect London dispersion forces, which are generally considered the weakest of the intermolecular forces. It is explained that these forces depend on the size and surface area of the molecules, as well as their polarizability. As you go down the periodic table, atoms become larger and more polarizable, leading to stronger temporary dipoles and London dispersion forces. The paragraph also touches on ion-dipole forces, which are strong interactions that occur in mixtures, such as when ionic compounds are dissolved in polar liquids. The strength of these forces depends on the polarity of the liquid, the charge and size of the ions, with smaller and more highly charged ions leading to stronger interactions.
🌡️ Impact of Intermolecular Forces on Physical Properties
The video discusses how intermolecular forces influence the physical properties of substances, particularly boiling points. It explains that stronger intermolecular forces require more energy to break, leading to higher boiling points, melting points, viscosity, and surface tension. An exception is vapor pressure, which is lower with stronger intermolecular forces because fewer molecules escape into the vapor phase. The paragraph provides a detailed comparison of boiling points based on the presence of hydrogen bonding, dipole-dipole forces, and London dispersion forces. It emphasizes that hydrogen bonding typically results in the highest boiling points, with dipole-dipole forces and size being the next considerations.
📉 Boiling Point Comparisons and Molecular Size
This part of the video script focuses on the comparison of boiling points and the role of molecular size in the context of intermolecular forces. It is explained that if no hydrogen bonding is possible, then the size of the molecule becomes a significant factor. Larger non-polar molecules can have stronger London dispersion forces that can surpass the dipole-dipole forces of smaller polar molecules, especially if the non-polar molecule is more than 15% larger. The script also addresses the impact of branching in hydrocarbons, noting that more branched, compact structures have lower surface areas and thus lower London dispersion forces, which can lead to lower boiling points compared to their unbranched counterparts with the same molecular weight.
🔄 Effects of Branching on Melting and Boiling Points
The video script explores the effects of branching in hydrocarbons on their physical properties, particularly melting and boiling points. It is often taught that branching lowers the boiling point but raises the melting point due to more compact structures that can pack into a crystal better. However, this trend is not always consistent, and the script advises that the melting point trend should be used with caution. The example provided ranks the boiling points of different hydrocarbons based on size and branching, with the smallest and least branched molecule having the highest boiling point. When considering melting points, the ranking can differ, especially with highly branched and symmetrical structures, which may have higher melting points than their unbranched counterparts.
💧 Solubility and the 'Like Dissolves Like' Principle
The final paragraph of the video script discusses solubility and the principle that 'like dissolves like,' meaning polar solutes dissolve in polar liquids, and non-polar solutes dissolve in non-polar liquids. It explains how to determine the solubility of different compounds in very polar liquids like water and very non-polar liquids like hexane. For water solubility, the focus is on choosing molecules with the most polarity and minimizing non-polar regions. Conversely, for hexane solubility, the choice is the molecule with the greatest non-polar character, typically indicated by the largest hydrocarbon chain. The video concludes by inviting viewers to like, share, and comment with questions, and to check out additional resources for practice problems and study guides.
Mindmap
Keywords
💡Intermolecular Forces
💡Hydrogen Bonding
💡Dipole-Dipole Forces
💡London Dispersion Forces
💡Ion-Dipole Forces
💡Polarity
💡Boiling Point
💡Melting Point
💡Surface Tension
💡Vapor Pressure
💡Viscosity
Highlights
Intermolecular forces are the focus of the last lesson in the first chapter of the organic chemistry playlist.
Hydrogen bonding, dipole-dipole forces, London dispersion forces, and ion-dipole forces are discussed.
Intermolecular forces are weaker than ionic and covalent bonds, with hydrogen bonding being the strongest among them.
Hydrogen bonding typically requires a hydrogen bonded to fluorine, oxygen, or nitrogen.
Dipole-dipole forces occur between polar molecules and are stronger in more polar molecules.
London dispersion forces are temporary dipoles that occur in all molecules but are the weakest of the three forces.
Ion-dipole forces are stronger than the other intermolecular forces but only occur in mixtures.
The size, surface area, and polarizability of molecules affect the strength of London dispersion forces.
Boiling points, melting points, surface tension, and viscosity are bulk properties affected by intermolecular forces.
Higher intermolecular forces lead to higher boiling and melting points, viscosity, and surface tension.
Vapor pressure is lower with higher intermolecular forces as it is harder for molecules to escape into the vapor phase.
When comparing boiling points, the presence of hydrogen bonding is a key factor.
Molecules with greater size and surface area have stronger London dispersion forces, leading to higher boiling points.
Branching in hydrocarbons leads to a more compact structure with lower surface area, reducing London dispersion forces.
The principle 'like dissolves like' governs solubility, meaning polar solutes dissolve in polar liquids, and non-polar in non-polar liquids.
For solubility in water, smaller hydrocarbon chains are preferred to minimize non-polar regions.
For solubility in non-polar solvents like hexane, larger hydrocarbon chains are preferred for more non-polar interactions.
Transcripts
Browse More Related Video

11.1 Intermolecular Forces | General Chemistry

10.1 Intermolecular Forces | High School Chemistry

Van der Waals forces | States of matter and intermolecular forces | Chemistry | Khan Academy
Intermolecular Forces and Boiling Points
AP Chemistry Unit 2 Review

AP Chem - Unit 3 Review - Intermolecular Forces & Properties
5.0 / 5 (0 votes)
Thanks for rating: