[H2 Chemistry] 2023 Topic 2 Chemical Bonding 1
TLDRThis chemistry lecture introduces advanced concepts of chemical bonding, emphasizing the electrostatic nature of all bonds and challenging common misconceptions from secondary school. It covers the fundamentals of metallic, ionic, and covalent bonding, including the role of electronegativity, lattice energy, and coordination numbers. The lecture also explores the physical properties of metals and ionic compounds, such as melting points, electrical conductivity, and hardness, providing a deeper understanding of chemical intuition and the factors influencing bond strength.
Takeaways
- 🧪 Discard secondary school chemical bonding knowledge and approach the topic with a fresh perspective.
- 📚 The topic of chemical bonding will take 3-4 weeks, but full mastery may take months.
- 🔄 All forms of chemical bonding are electrostatic in nature, including covalent, ionic, and metallic bonds.
- 🧲 Ionic bonding involves the attraction between positively charged cations and negatively charged anions.
- 🔗 Covalent bonding involves sharing of electrons, with the electrostatic attraction between shared electrons and nuclei.
- 📊 The concept of hybridization will be introduced early to facilitate understanding in organic chemistry.
- 🔍 Instantaneous dipole-induced dipole interactions, also known as London dispersion forces, are a type of intermolecular force.
- 🔋 Metals are good conductors of electricity due to the presence of delocalized electrons.
- 💡 The strength of metallic bonding is related to the number of valence electrons and the charge density of metal cations.
- 🌐 Ionic compounds tend to have high melting and boiling points due to strong electrostatic forces between ions.
Q & A
Why should students discard their previous knowledge of chemical bonding from secondary school?
-Students should discard their previous knowledge because the approach to chemical bonding in higher education is very different, and preconceived notions from secondary school might impede their understanding of the more complex concepts introduced at the university level.
How long is the typical duration to cover the topic of chemical bonding?
-The topic of chemical bonding usually spans around three to four weeks of lectures and exercises.
Why is it not expected for students to achieve perfect mastery of chemical bonding within three to four weeks?
-Achieving perfect mastery is not expected because chemical bonding is a complex subject that requires time to develop chemical intuition, which can take a few months to fully appreciate.
What is the fundamental nature of all forms of chemical bonding?
-All forms of chemical bonding are electrostatic in nature, involving the attractive forces between positively and negatively charged particles.
Why might the representation of atoms and ions as spheres in drawings be misleading?
-The representation of atoms and ions as spheres can be misleading because they are not tangible objects but rather electron clouds, and the drawings do not reflect the actual scale or distances between particles in reality.
What is the relationship between electronegativity values and the type of chemical bond formed?
-Similar electronegativity values tend to form covalent bonds, while a large difference in electronegativity values leads to ionic bonding due to the transfer of electrons from one atom to another.
Why are electronegativity values not provided in the data booklets during exams?
-Electronegativity values are not provided because students are expected to understand the general trends of electronegativity across the periodic table rather than memorize specific values.
What are the factors that affect the strength of ionic bonding?
-The strength of ionic bonding is affected by factors such as the charge of the ions, the size of the ions (ionic radii), and the lattice energy, which is directly proportional to the product of the charges and inversely proportional to the sum of the ionic radii.
Why do metals exhibit high melting and boiling points?
-Metals have high melting and boiling points due to the strong and extensive metallic bonds within their structure, which require a significant amount of energy to overcome.
What is the reason behind the malleability and ductility of metals?
-Metals are malleable and ductile because the non-directional metallic bonding allows the layers of atoms to slide past each other without breaking the bond, as long as the sea of delocalized electrons remains surrounding the cations.
Why are ionic compounds non-conducting in the solid state but conducting when molten or in aqueous solution?
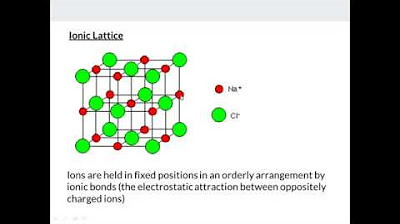
-Ionic compounds are non-conducting in the solid state because the ions are in fixed positions. They become conducting when molten or in aqueous solution due to the presence of free mobile ions that can move in response to a potential difference.
Outlines
📚 Introduction to Chemical Bonding
The script introduces the topic of chemical bonding, emphasizing its complexity and advising students to discard preconceived notions from secondary school. It highlights the importance of developing chemical intuition over time, rather than expecting immediate mastery. The lecture will cover a range of subtopics, including hybridization, and stresses that all chemical bonds are fundamentally electrostatic in nature, which might be a new concept for students. The script also clarifies that while covalent bonds involve electron sharing, the attraction in these bonds is also electrostatic due to the nuclei's attraction to the electron pairs.
🔬 Understanding Intramolecular and Intermolecular Forces
This section delves into the differences between intramolecular forces, such as ionic, covalent, and metallic bonds, which are strong interactions within a molecule or lattice, and intermolecular forces, which include dipole-dipole interactions and hydrogen bonding. The script corrects common misconceptions about how atoms and ions are represented and emphasizes the limitations of these representations. It also introduces the concept of electronegativity and how it affects the distribution of electron density within bonds.
📉 Trends in Electronegativity and Chemical Bonding
The script discusses how electronegativity values increase across a period and decrease down a group in the periodic table, affecting the type of chemical bonds formed. It advises students to remember the electronegativity values of hydrogen, fluorine, oxygen, and nitrogen, as these elements are particularly important for understanding chemical bonding. The section also explains that similar electronegativity values tend to result in covalent bonds, while a large difference leads to ionic bonds.
🌟 Transition Elements and Their Bonding Nature
This part of the script explores the bonding nature of transition elements, which can form both ionic and covalent bonds. It explains that while Group 1 and 2 elements typically form ionic bonds with non-metals, transition elements with higher electronegativity values can form more covalent-like bonds. The script encourages students not to rush their understanding of these concepts and to focus on grasping the fundamentals of the different types of bonding.
🔑 Periodic Trends in Intra-Molecular Bonding
The script examines the trends in intra-molecular bonding across period three of the periodic table, showing the transition from metallic to non-metallic character and the corresponding change in structure from giant metallic to giant molecular to simple molecular. It also notes that noble gases, such as argon, are monoatomic and do not typically form covalent bonds due to their stable electron configurations.
🔍 Exercise 1.1: Identifying Types of Chemical Bonding
This section presents an exercise to identify the types of chemical bonding in various substances, such as barium, oxygen, barium oxide, and nitrogen dioxide. It guides students in using the periodic table to determine whether the bonding is metallic, covalent, or ionic based on the position of the elements and their properties. The script reinforces the idea that most chemical bonds are not purely covalent or ionic but fall somewhere on a spectrum between the two.
🌐 Metallic Bonding and Its Characteristics
The script provides an overview of metallic bonding, describing it as non-directional due to the free movement of delocalized electrons within a metallic lattice. It explains that the strength of metallic bonds is related to the number of valence electrons per metal atom and the charge density of the metal cations. The section also discusses the physical properties of metals, such as high melting and boiling points, and their ability to conduct electricity and heat.
🔧 The Malleability and Ductility of Metals
This part of the script discusses the malleability and ductility of metals, explaining that these properties arise from the non-directional nature of metallic bonds and the strong attraction between the delocalized electrons and metal cations. It also touches on the hardness of metals and how alloying can increase this hardness by preventing layers of metal from sliding past each other easily.
💎 Ionic Bonding and the Properties of Ionic Compounds
The script introduces ionic compounds, using sodium chloride as an example, and explains the misconception that each ion forms a limited number of ionic bonds. It clarifies that ionic bonding is non-directional and extends infinitely in all directions. The section also covers coordination numbers and the factors that influence them, such as the size and charge of ions.
🔗 Lattice Energy and Its Relation to Ionic Bond Strength
This section delves into lattice energy, which is used as a proxy for the strength of ionic bonds. It explains that lattice energy is the heat evolved when an ionic compound is formed from its constituent gaseous ions and is directly proportional to the product of the charges and inversely proportional to the sum of the ionic radii. The script emphasizes the importance of understanding this relationship when comparing the strength of ionic bonds in different compounds.
🔥 Physical Properties of Ionic Compounds
The script outlines the physical properties of ionic compounds, such as their high melting and boiling points, which result from the strong ionic bonds. It also discusses the electrical conductivity of ionic compounds, which is dependent on the presence of free mobile ions in the molten or aqueous state. Additionally, it touches on the hardness and rigidity of ionic compounds and the factors that affect their solubility.
Mindmap
Keywords
💡Chemical Bonding
💡Electrostatic Forces
💡Covalent Bonding
💡Ionic Bonding
💡Metallic Bonding
💡Electronegativity
💡Lattice Energy
💡Coordination Number
💡Intramolecular and Intermolecular Forces
💡Hybridization
💡Energetics
Highlights
The lecture emphasizes the importance of discarding preconceived notions from secondary school to fully embrace the complexity of chemical bonding.
Chemical bonding is introduced as an electrostatic phenomenon, differing from common secondary school explanations, especially for metallic and covalent bonds.
The concept of hybridization is introduced early in the curriculum to facilitate easier understanding of organic chemistry topics later on.
The development of 'chemical intuition' is highlighted as a gradual process that takes months, rather than weeks, to fully appreciate.
Learning outcomes span multiple topics, including energetics, organic chemistry, and atomic structure, indicating the interdisciplinary nature of chemical bonding.
The representation of atoms and ions in diagrams is discussed, with a caution against misunderstanding them as tangible spheres.
Intramolecular bonding, such as covalent, ionic, and metallic, is differentiated from intermolecular forces like ion-dipole and hydrogen bonding.
Electronegativity values are introduced as a key factor in determining the nature of chemical bonds, with trends across the periodic table explained.
The significance of electronegativity in understanding the polarity of bonds and the formation of ionic versus covalent bonds is discussed.
The periodic table is used to predict the type of chemical bonding in elements, with examples provided for barium, oxygen, and nitrogen dioxide.
Metallic bonding is characterized by its non-directional nature due to the free movement of delocalized electrons.
The strength of metallic bonding is related to the number of valence electrons and the charge density of metal cations.
Physical properties of metals, such as high melting and boiling points, are attributed to the strong and extensive metallic bonds.
Metals are described as good conductors of electricity and heat due to the presence of mobile charge carriers.
Malleability and ductility of metals are explained by the non-directional nature of metallic bonds and the strong attraction between delocalized electrons and cations.
The hardness of metals and the use of alloys to increase it is discussed, with the role of different metal atoms preventing layers from sliding past each other.
Ionic compounds are introduced with sodium chloride as a model, emphasizing the non-directional nature of ionic bonds and the concept of coordination numbers.
Lattice energy is defined and its relationship with the strength of ionic bonding is explained, with the greater the lattice energy indicating stronger ionic bonds.
The physical properties of ionic compounds, such as high melting and boiling points, are linked to the strength of ionic bonds and lattice energy.
Electrical conductivity in ionic compounds is discussed, noting that it only occurs in molten or aqueous states due to the presence of free mobile ions.
The hardness and rigidity of ionic compounds are explained, with the repulsion of like charges causing the crystal to fracture when forces are applied.
The solubility of ionic compounds is touched upon, with a reference to more detailed explanations in later topics on energetics and thermodynamics.
Transcripts
Browse More Related Video

8.1 Ionic, Covalent, and Metallic Bonding | High School Chemistry
Chemical Bonding Concepts (Part 2)

[H2 Chemistry] 2023 Topic 2 Chemical Bonding 2

Ionic Bonding [IB Chemistry SL/HL]

Energy, Ionic Solids, Metals, & Alloys - AP Chem Unit 2, Topics 2-4

AP Chem - Unit 2 Review - Molecular & Ionic Compound Structure and Properties
5.0 / 5 (0 votes)
Thanks for rating: