Electric Charge, Law of Charges, and Quantization of Charge
TLDRThe video script explores the concept of electric charge, demonstrating through experiments with a rubber balloon that charges can be positive or negative and are quantized in units of the elementary charge. It explains the law of charges, stating that like charges repel and unlike charges attract, and delves into the fundamental particles responsible for charge, such as protons and electrons, highlighting their properties and interactions.
Takeaways
- 🎈 Rubbing a rubber balloon against hair can cause the balloon to stick due to the transfer of electrons, resulting in an excess negative charge on the balloon.
- 🔋 The law of charges states that unlike charges (positive and negative) attract each other, while like charges (positive-positive or negative-negative) repel each other.
- 🧪 When a rubber rod is rubbed against fur, it acquires an excess negative charge, and when brought near a negatively charged balloon, they repel each other.
- 🏹 A glass rod rubbed against silk gains an excess positive charge, and when brought near a negatively charged balloon, they are attracted to each other.
- 🔋 The concept of positive and negative charges is attributed to Benjamin Franklin and is a convention rather than an inherent property of the charges themselves.
- 📈 The elementary charge (approximately 1.60 x 10^-19 Coulombs) is the smallest measured charge on an isolated particle and is the fundamental unit of electric charge.
- ⚛️ Electrons and protons are subatomic particles with charges of equal magnitude but opposite signs; electrons carry a negative charge, while protons carry a positive charge.
- 🚩 Protons are not elementary particles and are composed of quarks, specifically two up quarks and one down quark, giving them a net positive charge.
- 📐 The mass of an electron is significantly less than that of a proton, with the electron being about 1/2000th the mass of a proton.
- 🔢 Electric charge is quantized, meaning that the net charge on an object must be an integer multiple of the elementary charge, as charges cannot exist in fractional amounts.
- 🔧 The concept of electric force, which is related to charge, will be defined and explored in more detail in subsequent lessons.
Q & A
What happens when a rubber balloon is rubbed against the head?
-The balloon sticks to the head, some hair stands on end, and the balloon can later stick to the wall due to the transfer of electrons and the resulting static charge.
What is the law of charges?
-The law of charges states that unlike charges attract and like charges repel each other.
What does it mean for an object to be positively or negatively charged?
-An object is positively charged when it has more protons than electrons, and negatively charged when it has more electrons than protons.
Who attributed the terms 'positive' and 'negative' to electric charges?
-Benjamin Franklin attributed the terms 'positive' and 'negative' to electric charges.
What is the elementary charge?
-The elementary charge, approximately 1.60 x 10^-19 Coulomb's, is the smallest measured charge on an isolated particle, represented by the symbol 'e'.
What are the basic properties of electrons and protons?
-Electrons carry a negative charge with a mass of 9.11 x 10^-31 kilograms, while protons carry a positive charge with a mass of 1.67 x 10^-27 kilograms, which is almost 2,000 times greater than that of an electron.
Why are protons not considered elementary particles?
-Protons are not considered elementary particles because they are composed of smaller particles called quarks, specifically two up quarks and one down quark.
How does the transfer of electrons during rubbing result in an excess charge?
-When objects are rubbed together, electrons can move from one material to another. The object gaining electrons becomes negatively charged, while the object losing electrons becomes positively charged.
What is the significance of charge quantization?
-Charge quantization means that charge can only exist in integer multiples of the elementary charge. This is because charged particles like electrons and protons cannot be divided into smaller parts.
What happens when the wall becomes polarized and how does it relate to the balloon sticking to it?
-Polarization of the wall occurs when the electrons in the wall are displaced due to the presence of the charged balloon. This creates a separation of charge within the wall, leading to an attractive force between the wall and the balloon, causing the balloon to stick.
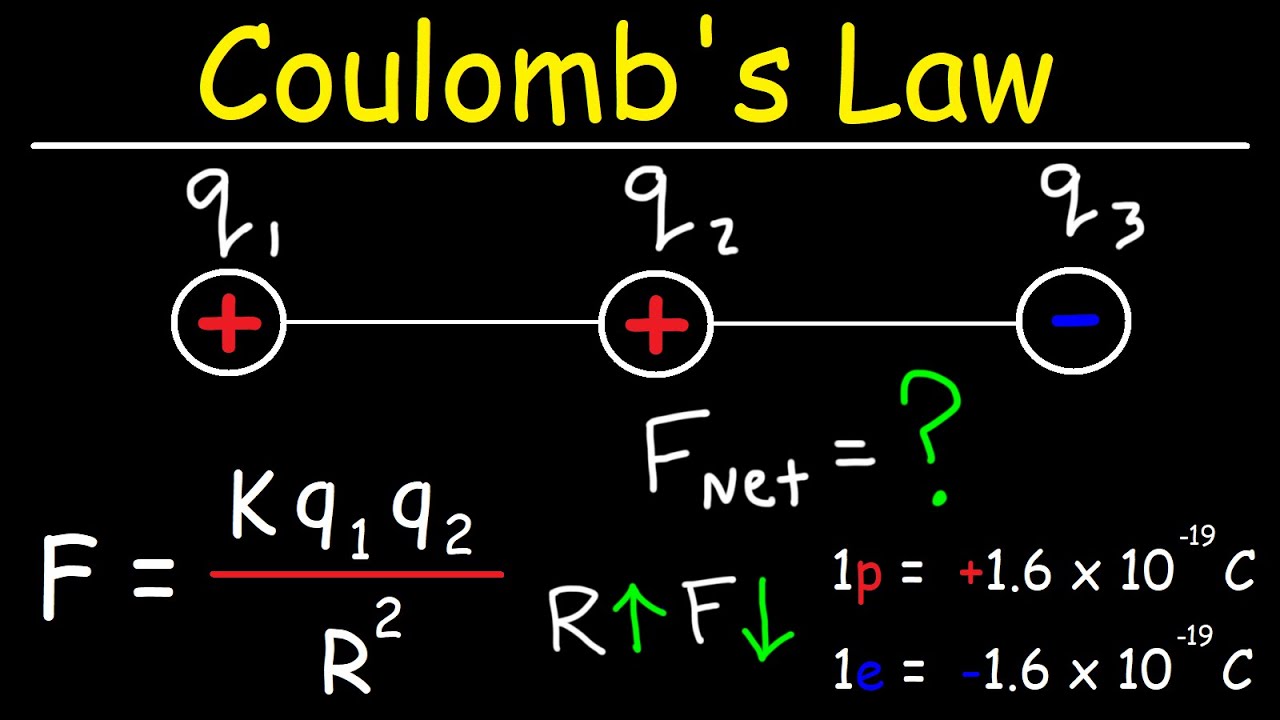
How many excess protons would it take to have an excess charge of one Coulomb?
-It would take 6.25 x 10^18 excess protons to have an excess charge of one Coulomb, as the charge on an object is the result of the number of excess charge carriers (protons in this case) times the elementary charge.
What is the definition of electric charge in the context of physics?
-Electric charge is the physical property of matter that causes it to experience an electric force. It is quantized and can be positive or negative, with protons having a positive charge and electrons having a negative charge.
Outlines
🔬 Introduction to Electric Charges
This paragraph introduces the concept of electric charges through a simple demonstration involving a rubber balloon. It explains the phenomenon of the balloon sticking to the head and the wall after being rubbed, and how this relates to the law of charges. The discussion delves into the nature of charge, mentioning protons and electrons, and the positive or negative nature of charge. The paragraph sets the stage for further exploration of charges by using the balloon as an example to understand how charges can be transferred between objects, leading to an excess of negative or positive charges.
🧪 Experiments with Charges and Coulomb's Law
This paragraph continues the exploration of electric charges with more experiments and introduces Coulomb's Law. It describes how rubbing different materials can result in the transfer of electrons, leading to objects having either an excess negative or positive charge. The discussion also touches on the concept of elementary charge and its significance. The paragraph further explains the mass and charge differences between electrons and protons, and introduces the composition of protons as being made up of quarks. The explanation of how charges interact with each other, and the importance of the law of charges in understanding these interactions, is emphasized.
📐 Quantization of Charge and Understanding Elementary Particles
The final paragraph delves into the quantization of electric charge, explaining that charge comes in discrete quantities, which are multiples of the elementary charge. It clarifies that objects cannot have a fraction of a charge, as charges are quantized. The paragraph also addresses the impossibility of having a net charge that is not an integer multiple of the elementary charge. The discussion includes the calculation of the number of protons needed to achieve a one Coulomb charge, highlighting the immense number involved. The paragraph concludes with a recap of the key points about electric charge, emphasizing its role as a physical property of matter that causes objects to experience an electric force, although the precise definition of electric force is reserved for a future lesson.
Mindmap
Keywords
💡Charge
💡Protons
💡Electrons
💡Coulomb
💡Quantized
💡Law of Charges
💡Elementary Charge
💡Quarks
💡Polarization
💡Electric Force
Highlights
Rubbing a rubber balloon against your head causes it to stick and some hairs to stand on end.
The rubber balloon gains the ability to stick to walls after being rubbed on the head.
Introduction of basic principles of electrical charges, including the attraction and repulsion between charged objects.
Demonstration with a rubber balloon and a rubber rod shows that like charges repel.
A contrasting experiment with a glass rod and silk illustrates that unlike charges attract.
Explanation of why the rubber balloon and rubber rod repel each other due to both having an excess negative charge.
Discussion of Benjamin Franklin’s naming of positive and negative charges.
Clarification that objects are not inherently charged positively or negatively; rather, they have an excess of electrons or a deficit, leading to negative or positive charge respectively.
Exploration of how the transfer of electrons between objects results in changes in their charge states.
Introduction to the elementary particles involved in electrical charges, protons, and electrons.
Explanation of the role of quarks in the composition of protons and neutrons.
Clarification that a Coulomb is the SI unit for measuring electric charge.
Discussion on the immensity of the force between two objects each having a charge of one Coulomb.
Calculations showing the number of excess protons required to achieve a net charge of one Coulomb.
Explanation of the quantization of charge, stating that charge must be an integer multiple of the elementary charge.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: