What Is Charge?
TLDRThe script introduces the concept of electric charge, explaining that matter is composed of charged particles: electrons with a negative charge and protons with a positive charge. It emphasizes that charge is quantized, meaning it comes in integer multiples of the elementary charge (e), and conserved, indicating that the total charge in a system remains constant. The demonstration shows that like charges repel and unlike charges attract, and that charging an object involves separating positive and negative charges. The script also hints at the deeper complexity of charge within protons and the role of quarks.
Takeaways
- 🔋 Charge is a fundamental property of matter composed of particles with positive and negative electrical charges - protons and electrons respectively.
- ⚖️ The unit of electric charge is the coulomb, which is a large unit requiring 1.6 × 10^19 electrons to make up a single coulomb.
- 🔢 Charge is quantized, meaning that the charge of any object is an integer multiple of the elementary charge (e), reflecting the discrete nature of protons and electrons.
- 🔄 Despite the quantization of charge, inside protons, there are quarks with fractional charges (1/3 e or -2/3 e), but these are always confined within protons and do not affect the overall charge.
- 🧪 The quantization of charge is an experimental fact, with no known deeper explanation, and it is regularly tested for precision and accuracy by experimental physicists.
- 🔄 Charge conservation is a fundamental principle stating that the total charge in an isolated system remains constant; charge cannot be created or destroyed.
- 💥 Like charges repel each other, while opposite charges attract, a key principle demonstrated through interactions between various materials and objects in the script.
- 🌟 When a charged object comes into contact with a conductor, such as a balloon, charge separation occurs within the conductor, leading to attraction or repulsion based on the charges involved.
- 🏷️ The convention of labeling charges is such that the charge of a glass rod rubbed with fur is considered positive, while the charge of a rubber rod rubbed with silk is considered negative.
- 🔬 Simple experiments, like rubbing a rod with fur or bringing charged objects near tinsel or balloons, can effectively illustrate the principles of charge conservation, quantization, and interaction.
Q & A
What is the fundamental composition of matter in terms of charge?
-Matter is composed of charged particles, specifically electrons with a negative charge and protons with a positive charge.
What is the unit of electric charge?
-The unit of electric charge is the coulomb, which is a significant amount of charge that takes 1.6 times 10 to the 19 electrons to make.
How is the charge of an electron represented?
-The charge of a single electron is represented as minus e, indicating its negative charge.
What does it mean for charge to be quantized?
-Charge being quantized means that the charge of any object is an integer multiple of the elementary charge (e). It can be -e, +e, -27e, +53e, etc.
Why do we say charge is quantized, despite the presence of quarks inside protons with fractional charges?
-We say charge is quantized because quarks inside protons are always bound together and do not exist freely. Thus, when not considering the internal structure of protons, the charge is still quantized in units of e.
What is the experimental fact about charge quantization?
-The experimental fact about charge quantization is that the charge of the electron is exactly minus the charge of the proton, and this is consistently verified through precise experiments.
What does the conservation of charge imply?
-The conservation of charge implies that the total amount of charge in an isolated system remains constant over time. Charge cannot be created or destroyed, only transferred from one object to another.
How does one object become positively charged?
-An object becomes positively charged by losing electrons, which are negatively charged. This is often done by rubbing the object with another material, causing electron transfer.
What is the third basic fact about charge demonstrated in the script?
-The third basic fact about charge is that like charges repel each other, while opposite charges attract.
How does a charged rod interact with a neutral balloon?
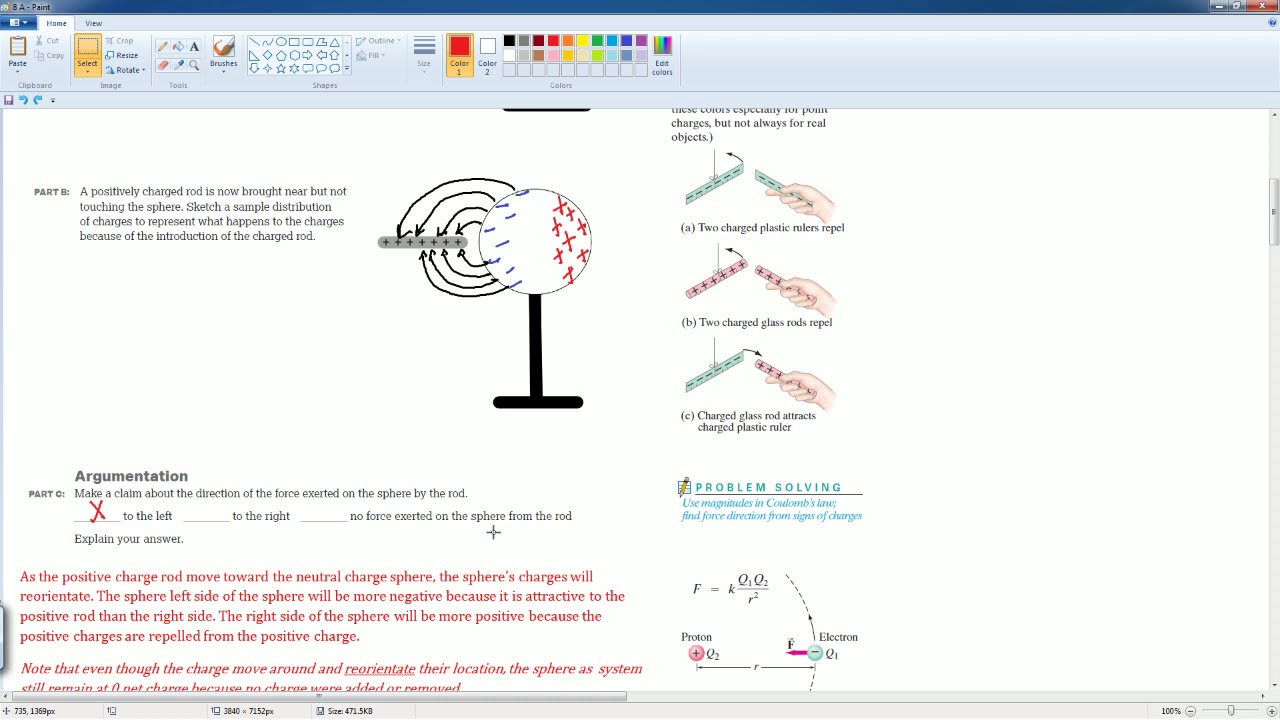
-A charged rod can attract a neutral balloon because the charge on the rod induces a separation of charge within the balloon, creating a negative side that is attracted to the positive rod.
What property of the balloon allows it to be attracted by the charged rod?
-The balloon is a conductor, which allows it to separate charge when in proximity to a charged object, leading to an attraction between the induced charges and the charged rod.
Outlines
🔋 Understanding the Basics of Charge
This paragraph introduces the fundamental concept of charge, explaining that all matter is composed of charged particles: electrons with a negative charge and protons with a positive charge. It emphasizes that matter is typically neutral, having equal numbers of positive and negative charges. The unit of charge, the coulomb, is introduced, and it's noted that it takes a large number of electrons to make up one coulomb. The idea that charge is quantized, meaning it can only exist in integer multiples of the elementary charge (e), is discussed. The paragraph also touches on the fact that charge is conserved, and while it can be transferred from one object to another, it cannot be created or destroyed. The demonstration of like charges repelling and opposite charges attracting is set up, using tinsel and a charged rod as examples.
🔄 The Conservation of Charge in Action
This paragraph delves deeper into the concept of charge conservation, illustrating it with experiments. It explains that negative charge can be observed on the fur after being rubbed against a plexiglass rod, which becomes positively charged. The total charge remains the same, demonstrating conservation. The paragraph also describes how a neutral balloon, when brought near a positively charged rod, will have charge separated within it, leading to an attraction between the rod and the negative side of the balloon. This further reinforces the principles of charge conservation and interaction, showing that while the overall charge is conserved, it can be redistributed, leading to observable phenomena like attraction and repulsion.
Mindmap
Keywords
💡Charge
💡Quantized
💡Conserved
💡Coulomb
💡Electrons
💡Protons
💡Quarks
💡Repel
💡Attract
💡Neutral
💡Conductor
Highlights
All matter is composed of charged particles, electrons and protons.
Electrons have a negative charge, while protons have a positive charge.
Under normal conditions, matter is neutral, having equal amounts of positive and negative charges.
The unit of charge is the coulomb, which represents a significant quantity of charge.
It takes 1.6 times 10 to the 19 electrons to make one coulomb of charge.
Charge is quantized, meaning it can only exist in integer multiples of the elementary charge.
The charge of a proton is exactly opposite to that of an electron.
Inside a proton, there are quarks with charges of 1/3 e or -2/3 e.
Charge quantization is an experimental fact with no profoundly understood reason.
Experimental physicists regularly test the quantization of charge with high precision.
Charge conservation means that net charge cannot be created or destroyed.
Like charges repel each other, while opposite charges attract.
Charging an object involves separating positive and negative charges.
A charged object can attract a neutral object by inducing charge separation within it.
A neutral balloon can be attracted by a charged rod due to induced charge separation.
The conservation of charge is a fundamental principle in physics.
Simple demonstrations can effectively illustrate the principles of charge conservation and interaction.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: