BTEC Applied Science: Unit 1 Chemistry Inter-molecular Forces
TLDRThe video script delves into the concept of intermolecular forces, emphasizing forces that exist between molecules rather than within. It introduces van der Waals forces as the weakest, which arise due to temporary dipoles caused by uneven electron distribution. The script further explains permanent dipoles, highlighting their continuous attraction and the role of electronegativity in their formation. A special type of permanent dipole-dipole interaction, the hydrogen bond, is discussed, with a focus on its significance in maintaining liquid water, essential for life. The strength of these intermolecular forces is compared to covalent bonds, with hydrogen bonds being notably strong, followed by other permanent dipoles and the weakest van der Waals forces.
Takeaways
- 🔍 Intermolecular forces are the attractions between molecules, distinct from the forces within a molecule or covalent bonds.
- 🔐 Van der Waals forces are weak intermolecular forces that result from temporary dipoles in atoms or molecules.
- 💫 Electrons in atoms move rapidly and unevenly distributed, leading to temporary negative and positive ends, or dipoles.
- 🪜 Molecules with permanent dipoles have distinct negative and positive ends due to differences in electronegativity, leading to permanent dipole-dipole attractions.
- 🔗 Hydrogen bonding is a special type of permanent dipole-dipole interaction where hydrogen is bonded with highly electronegative elements like fluorine, oxygen, or nitrogen.
- 🌊 Water molecules (H2O) exhibit strong hydrogen bonding due to the large dipole between hydrogen and oxygen, which is crucial for life on Earth.
- 📈 The strength of intermolecular forces is significantly less than that of covalent bonds, with hydrogen bonds being the strongest among them.
- 📊 Compared to covalent bonds, hydrogen bonds are about 10 times weaker, other permanent dipole-dipole bonds are about 5 times weaker, and Van der Waals forces are the weakest, with a strength of about 1.
- 🌐 The existence of hydrogen bonds allows water to remain in liquid form at room temperature, which is essential for the existence of life.
- 🔑 Understanding the types and strengths of intermolecular forces is fundamental to explaining the properties and behaviors of substances.
- 📝 The three types of bonding between molecules are Van der Waals forces, permanent dipole-dipole interactions, and hydrogen bonds, with varying strengths and influencing factors such as molecular structure and electronegativity.
Q & A
What are intermolecular forces and how do they differ from forces within a molecule?
-Intermolecular forces are the attractions between molecules, as opposed to the forces within a molecule, such as covalent bonds, which involve the bonding of atoms within a single entity.
What is the first type of intermolecular force discussed in the transcript and how are they characterized?
-The first type of intermolecular force discussed is van der Waals forces, which are very weak forces that occur due to temporary dipoles in molecules.
How do temporary dipoles arise and lead to van der Waals forces?
-Temporary dipoles arise when electrons are not evenly distributed around an atom, leading to a temporary negative and positive end. These temporary dipoles can induce attraction between molecules for a very short period.
What is a permanent dipole and how does it differ from a temporary dipole?
-A permanent dipole is a consistent separation of positive and negative ends in certain molecules due to differences in electronegativity, unlike temporary dipoles which are fleeting and occur randomly.
How do permanent dipole-dipole interactions differ from van der Waals forces in terms of strength and duration?
-Permanent dipole-dipole interactions are stronger and last indefinitely as long as the molecules are in proximity, while van der Waals forces are weaker and temporary.
What is a hydrogen bond and how does it relate to permanent dipole-dipole interactions?
-A hydrogen bond is a special type of permanent dipole-dipole interaction that occurs when hydrogen is bonded to a highly electronegative atom like fluorine, oxygen, or nitrogen, resulting in a particularly strong attraction between molecules.
Why are hydrogen bonds crucial for the existence of life on Earth?
-Hydrogen bonds are essential because they allow water molecules to attract each other, leading to the formation of liquid water at room temperature, which is vital for life as we know it.
Compare the strength of covalent bonds to intermolecular forces such as hydrogen bonds, permanent dipole-dipole bonds, and van der Waals forces.
-If a covalent bond is assigned a strength of 100, hydrogen bonds, being particularly strong examples of permanent dipole-dipole interactions, have a strength of about 10, other permanent dipole-dipole bonds have a strength of about 5, and the very weak van der Waals forces have a strength of about 1.
What is electronegativity and how does it play a role in the formation of permanent dipoles?
-Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. It plays a role in the formation of permanent dipoles as molecules with atoms of different electronegativities will have a separation of charge, leading to a molecule with a negative and positive end.
How do the uneven distribution of electrons and the formation of temporary dipoles contribute to the overall stability of a substance?
-The uneven distribution of electrons and the formation of temporary dipoles contribute to the overall stability of a substance by allowing for weak intermolecular attractions that can adjust and reform as needed, which is crucial for the physical state and properties of the substance.
Can you provide an example of a substance that exhibits van der Waals forces and explain why it's significant?
-A noble gas like helium exhibits van der Waals forces. It's significant because despite being a monoatomic gas and not forming bonds, the weak van der Waals forces allow the atoms to condense into a liquid form at extremely low temperatures.
How do intermolecular forces influence the physical properties of a substance, such as boiling and melting points?
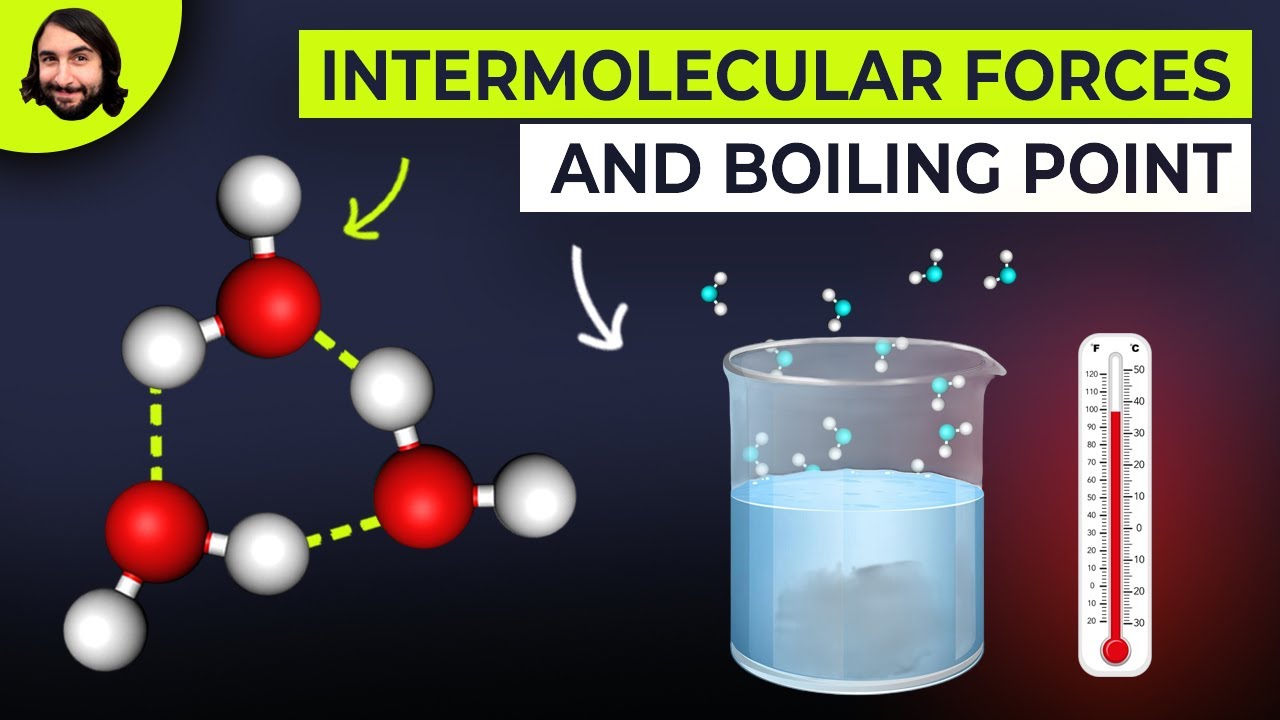
-Intermolecular forces greatly influence the physical properties of a substance. Stronger intermolecular forces result in higher boiling and melting points because more energy is required to overcome these attractions and separate the molecules.
Outlines
🔬 Introduction to Intermolecular Forces
This paragraph introduces the concept of intermolecular forces, emphasizing that these are the forces between molecules, distinct from the forces within a molecule such as covalent bonds. It raises the question of how molecules attract each other and what types of forces exist between them. The paragraph introduces the first type of intermolecular force, known as van der Waals forces or London forces, which are weak forces resulting from the uneven distribution of electrons around an atom, leading to temporary dipoles that attract other molecules for a short period.
Mindmap
Keywords
💡Intermolecular Forces
💡Van der Waals Forces
💡London Dispersion Forces
💡Permanent Dipole
💡Dipole-Dipole Interaction
💡Hydrogen Bond
💡Electronegativity
💡Molecular Attraction
💡Temporary Dipole
💡Strength of Bonds
Highlights
Intermolecular forces are the forces between molecules, distinct from the forces within a molecule such as covalent bonds.
Van der Waals forces, also known as London forces, are weak intermolecular forces that arise from temporary dipoles in atoms or molecules.
Electrons in an atom are not always evenly distributed, leading to temporary dipoles with a positive and negative end.
Molecules with permanent dipoles have a consistent positive and negative end, such as molecules with high electronegativity differences.
Permanent dipole-dipole attractions occur between molecules with permanent dipoles, such as in hydrogen chloride.
Hydrogen bonds are a special case of permanent dipole-dipole interaction, occurring when hydrogen is bonded with highly electronegative elements like fluorine, oxygen, or nitrogen.
Water molecules (H2O) have a large dipole and form hydrogen bonds, which are crucial for their liquid state at room temperature.
The existence of hydrogen bonds in water is fundamental for life on Earth, as it allows water to remain liquid, rather than being a gas at room temperature.
Comparing bond strengths, a covalent bond is much stronger than intermolecular forces, with hydrogen bonds being the strongest of the intermolecular forces.
Intermolecular forces play a significant role in determining the physical properties of substances, such as boiling and melting points.
Understanding intermolecular forces is essential for various scientific fields, including chemistry, physics, and materials science.
The concept of temporary and permanent dipoles is key to explaining the nature of intermolecular forces.
The uneven distribution of electrons and the formation of dipoles are dynamic processes that occur at the atomic and molecular level.
Electronegativity is a fundamental concept in understanding the polarity of molecules and the formation of permanent dipoles.
The strength of intermolecular forces can be quantified and compared to the strength of covalent bonds to understand the stability of molecular structures.
The study of intermolecular forces is vital for explaining phenomena such as solubility, phase transitions, and chemical reactions.
The presence of hydrogen bonds in biological molecules, like DNA and proteins, is critical for their structure and function.
The diversity of intermolecular forces contributes to the complexity and richness of chemical systems and reactions.
Exploring the nuances of intermolecular forces deepens our understanding of the physical world and the laws governing atomic and molecular interactions.
Transcripts
Browse More Related Video

Van der Waals forces | States of matter and intermolecular forces | Chemistry | Khan Academy

London Dispersion Forces | Chemistry
Intermolecular Forces and Boiling Points

10.1 Intermolecular Forces | High School Chemistry

1.6 Intermolecular Forces | Organic Chemistry

Intermolecular Forces - Hydrogen Bonding, Dipole Dipole Interactions - Boiling Point & Solubility
5.0 / 5 (0 votes)
Thanks for rating: