Intermolecular Forces - Hydrogen Bonding, Dipole Dipole Interactions - Boiling Point & Solubility
TLDRThis video delves into intermolecular forces, focusing on dipole-dipole interactions and hydrogen bonding. It explains how polar molecules like acetone and carbon monoxide engage in these interactions due to opposite charges. Hydrogen bonding, a special case of dipole-dipole interaction, is highlighted with water as an example, emphasizing its impact on boiling points and solubility. The video compares ethanol and dimethyl ether, and explains how the presence of hydrogen bonds and molecular structure influence boiling points and water solubility, contrasting small chain alcohols with larger, nonpolar molecules like octanol.
Takeaways
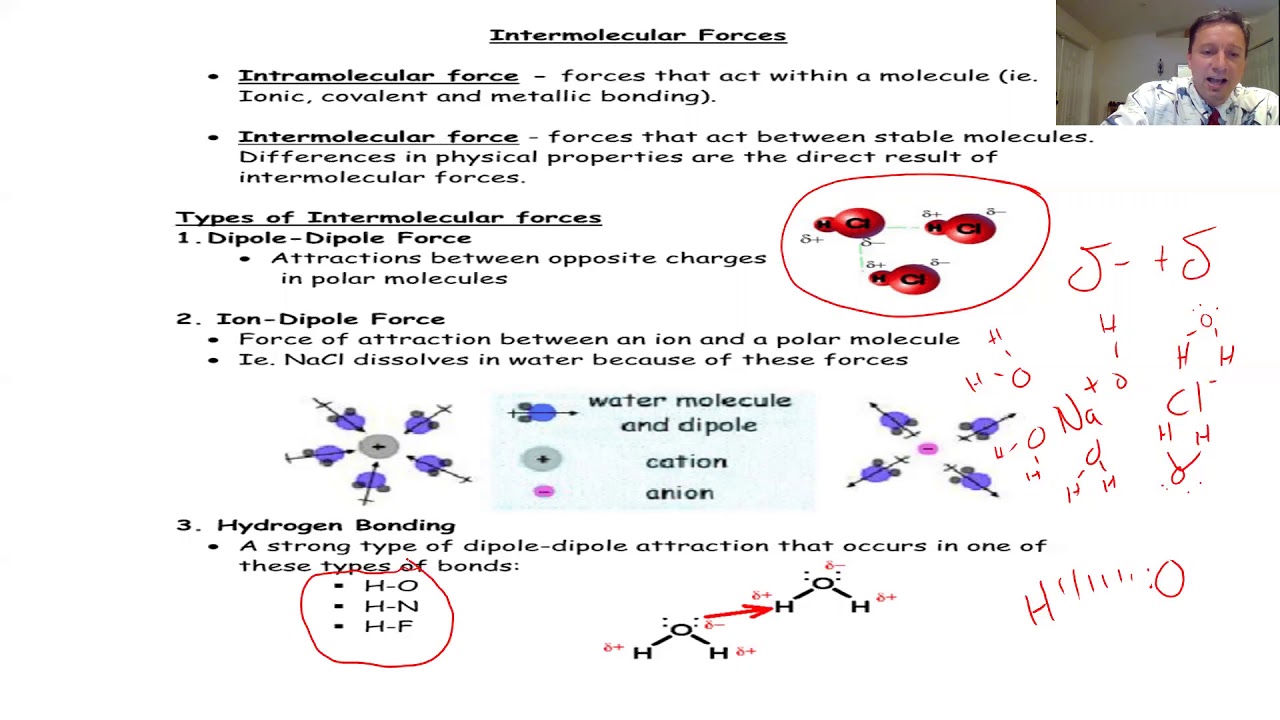
- 🧲 Dipole-dipole interactions occur between polar molecules, such as acetone, where the partially negative oxygen atom is attracted to the partially positive carbon atom of another molecule.
- 🔗 Carbon monoxide is an example of a molecule with a dipole moment, and the interaction between two such dipoles is known as a dipole-dipole interaction, which pulls them together due to opposite charges.
- 💧 Hydrogen bonding is a special type of dipole-dipole interaction that occurs when hydrogen is attached to nitrogen, oxygen, or fluorine, as seen in water molecules where the oxygen is attracted to the hydrogen of another molecule.
- 🌡 Hydrogen bonding significantly increases the boiling point of a molecule, as well as its water solubility, due to the strong intermolecular forces involved.
- 🌟 Examples like ammonia and methanol demonstrate increased solubility in water and higher boiling points due to the presence of hydrogen bonds.
- 🆚 Ethanol has a higher boiling point and greater water solubility than dimethyl ether because ethanol can form hydrogen bonds, while dimethyl ether, lacking direct hydrogen-oxygen bonds, cannot.
- 🔍 The comparison between ethanol and butanol shows that butanol, with a larger hydrocarbon chain, has a higher boiling point due to increased London dispersion forces, despite both being capable of hydrogen bonding.
- 🌊 The water solubility of butanol is less than that of ethanol due to the presence of a nonpolar hydrocarbon chain, which reduces its affinity for the polar water molecules.
- 🍺 Octanol, with its long nonpolar chain, is not soluble in water despite having hydrogen bonds, illustrating the dominance of nonpolar interactions in determining solubility.
- 🔑 Constitutional isomers like pentane and neopentane demonstrate that straight-chain alkanes have higher boiling points than branched isomers due to greater surface area and London dispersion forces.
- ⛓ The absence of OH groups in alkanes like pentane and neopentane means they do not dissolve in water, highlighting the importance of polar interactions for solubility.
Q & A
What are intermolecular forces?
-Intermolecular forces are the forces or interactions that occur between molecules, not necessarily within a molecule. They include dipole-dipole interactions, hydrogen bonding, and London dispersion forces, among others.
What is a dipole-dipole interaction?
-A dipole-dipole interaction occurs between polar molecules. It is an attraction between the positive end of one polar molecule and the negative end of another, causing them to be drawn together.
Why is acetone considered a polar molecule?
-Acetone is considered a polar molecule because it has a central carbon atom double-bonded to an oxygen atom, which has two lone pairs. This results in a separation of charge, with the oxygen atom having a partial negative charge and the carbon atom having a partial positive charge.
How does hydrogen bonding differ from regular dipole-dipole interactions?
-Hydrogen bonding is a special type of dipole-dipole interaction that occurs when hydrogen is attached to highly electronegative elements like nitrogen, oxygen, or fluorine. It is stronger than regular dipole-dipole interactions due to the small size and high charge density of the hydrogen atom.
What is the effect of hydrogen bonding on the boiling point of a molecule?
-Hydrogen bonding increases the boiling point of a molecule because it strengthens the intermolecular forces, requiring more energy to separate the molecules.
Why is water a good example of hydrogen bonding?
-Water is a good example of hydrogen bonding because the oxygen atom in a water molecule has a partial negative charge and the hydrogen atoms have a partial positive charge, allowing the oxygen of one water molecule to be attracted to the hydrogen of another.
How does the presence of hydrogen bonds affect the solubility of a molecule in water?
-The presence of hydrogen bonds increases the solubility of a molecule in water because it allows for stronger interactions between the polar solute and the polar solvent.
What is the difference between ethanol and dimethyl ether in terms of intermolecular forces?
-Ethanol has hydrogen bonds due to its OH group, which makes it highly polar and soluble in water, while dimethyl ether, despite being polar due to its bent shape and oxygen atom, lacks hydrogen bonds because hydrogen is not directly attached to oxygen.
Why does butanol have a higher boiling point than ethanol?
-Butanol has a higher boiling point than ethanol because, in addition to hydrogen bonds, it has more London dispersion forces due to its larger hydrocarbon chain, which increases the intermolecular forces.
How does the size of the hydrocarbon chain affect the solubility of alcohols in water?
-The size of the hydrocarbon chain affects the solubility of alcohols in water because a larger nonpolar hydrocarbon chain reduces solubility. Small chain alcohols like ethanol are highly soluble, but as the chain lengthens, the solubility decreases.
Why are straight-chain alkanes like pentane more soluble in water than branched alkanes like neopentane?
-Straight-chain alkanes like pentane are more soluble in water than branched alkanes like neopentane because straight-chain alkanes have more surface area and can form more London dispersion forces, which are a type of intermolecular force.
Outlines
🔬 Dipole-Dipole Interactions and Hydrogen Bonding
This paragraph discusses intermolecular forces, focusing on dipole-dipole interactions and hydrogen bonding. The video script uses acetone and carbon monoxide as examples to illustrate how polar molecules with partial charges can attract each other, forming dipole-dipole interactions. Hydrogen bonding is highlighted as a special case of this interaction, occurring when hydrogen is bonded to nitrogen, oxygen, or fluorine, with water as a key example. The script explains how hydrogen bonds affect the properties of molecules, such as increasing their boiling points and water solubility, using ethanol, dimethyl ether, and methanol as comparisons to demonstrate these effects.
🌡 Boiling Points and Solubility in Water
The second paragraph delves into the impact of hydrogen bonds and molecular structure on a substance's boiling point and solubility in water. It contrasts ethanol and dimethyl ether, noting ethanol's higher boiling point and solubility due to its hydrogen bonds. The discussion extends to comparing ethanol with butanol, highlighting how the size of the hydrocarbon chain affects these properties, with butanol having a higher boiling point due to additional London dispersion forces but lower solubility in water due to its larger nonpolar region. The paragraph also covers the solubility of alcohols with increasing hydrocarbon chain length, using octanol as an example to show decreased water solubility with a more extensive nonpolar region. It concludes with a comparison between pentane and neopentane, constitutional isomers with the same molecular formula but different structures, affecting their boiling points.
🚫 Nonpolar Molecules and Their Interactions with Water
The final paragraph briefly addresses the behavior of nonpolar molecules, such as methane, ethane, and propane, which do not form hydrogen bonds and therefore do not mix with water. This summary emphasizes the fundamental difference in intermolecular interactions between nonpolar molecules and those capable of forming hydrogen bonds, highlighting the lack of miscibility with water for nonpolar compounds.
Mindmap
Keywords
💡Intermolecular forces
💡Dipole-dipole interactions
💡Hydrogen bonding
💡Polar molecules
💡Boiling point
💡Water solubility
💡Ethanol
💡Dimethyl ether
💡London dispersion forces
💡Constitutional isomers
💡Nonpolar molecules
Highlights
Dipole-dipole interactions occur between polar molecules, such as acetone, where the oxygen atom has a partial negative charge and the carbon atom has a partial positive charge.
Dipole-dipole interactions are forces between molecules, not within a single molecule.
Carbon monoxide is an example of a polar molecule with a dipole moment, leading to dipole-dipole interactions.
Hydrogen bonding is a special type of dipole-dipole interaction that occurs when hydrogen is attached to nitrogen, oxygen, or fluorine.
Water molecules form hydrogen bonds due to the partial charges on oxygen and hydrogen, which increases their boiling point and water solubility.
Ammonia and methanol are examples of polar molecules with hydrogen bonds, resulting in high solubility in water.
Ethanol has a higher boiling point and water solubility than dimethyl ether due to its hydrogen bonds.
The boiling point and water solubility of molecules are influenced by the strength of intermolecular forces, such as hydrogen bonds.
Ethanol and butanol comparison shows that butanol has a higher boiling point due to additional London dispersion forces from its larger hydrocarbon chain.
Ethanol is more soluble in water than butanol due to its smaller nonpolar region compared to butanol's bulky nonpolar chain.
Octanol, with its large nonpolar region, is not soluble in water despite having hydrogen bonds.
Small chain alcohols like ethanol and methanol are highly soluble in water, while the addition of CH groups decreases solubility.
Pentane has a higher boiling point than neopentane due to its straight chain structure, which allows for more London dispersion forces.
Straight-chain alkanes have higher boiling points than branched alkanes due to increased surface area and intermolecular forces.
Molecules containing only carbon and hydrogen, like pentane and neopentane, are nonpolar and insoluble in water.
The structure of molecules, including the presence of polar bonds and hydrogen bonding, significantly affects their physical properties such as boiling point and solubility.
Transcripts
Browse More Related Video

Boiling Point of Organic Compounds

10.1 Intermolecular Forces | High School Chemistry

1.6 Intermolecular Forces | Organic Chemistry

AP Chem - Unit 3 Review - Intermolecular Forces & Properties
AP Chemistry Unit 2 Review

Van der Waals forces | States of matter and intermolecular forces | Chemistry | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: