Spontaneous Processes
TLDRThe video script discusses spontaneous processes in chemistry, emphasizing that these occur without external energy. It explains that a decrease in enthalpy (internal energy) and an increase in entropy (randomness) typically indicate a spontaneous reaction. However, it also introduces the concept of the equilibrium constant to determine whether a reaction favors products or reactants. The script uses examples like the rusting of iron and the formation of diamond from graphite to illustrate these principles, highlighting that some spontaneous processes may take a very long time to occur without external conditions.
Takeaways
- 📉 Spontaneous processes occur without external energy and tend to move in one direction.
- 🔄 The terms enthalpy and internal energy are used interchangeably in the context of spontaneous reactions.
- ⏬ A spontaneous process is indicated by a decrease in enthalpy and an increase in entropy.
- 🔄 Non-spontaneous processes are characterized by an increase in enthalpy and a decrease in entropy.
- ⚖️ The equilibrium constant (K) helps determine if a reaction favors products or reactants; K > 1 favors products, K < 1 favors reactants.
- 🕰️ Some spontaneous reactions may take a long time to occur, such as the formation of diamonds from graphite.
- 🔄 It's important to consider both enthalpy and entropy when assessing the spontaneity of a reaction.
- 🌡️ Changes in entropy can be observed as the dispersal or concentration of matter without a change in temperature.
- 🧪 The rusting of iron and the thermite reaction are examples of spontaneous reactions due to negative enthalpy changes.
- 🧊 Cold packs are an example of endothermic processes where entropy is consumed, resulting in a positive change in enthalpy.
- 🔄 Understanding Gibbs Free Energy is necessary to predict spontaneity in reactions that do not fit neatly into the categories of high or low enthalpy and entropy changes.
Q & A
What are spontaneous processes in chemistry?
-Spontaneous processes are those that occur without the need for external energy. They are reactions that happen naturally and move in one direction without needing to be driven by external forces.
How does the video illustrate a spontaneous process?
-The video illustrates a spontaneous process by showing the knocking over of a tower. The tower falling is a spontaneous process as it only requires a small amount of initial energy to initiate and then continues without further external input.
What is the relationship between enthalpy and spontaneous processes?
-In the context of spontaneous processes, a decrease in enthalpy, which indicates a decrease in internal energy, is often a sign of a spontaneous reaction. It suggests that the reactants have more energy than the products, favoring the formation of products.
How does entropy play a role in determining spontaneity?
-Entropy is a measure of the randomness or dispersal of matter. An increase in entropy, which indicates a greater dispersal of particles, also favors spontaneous processes. A spontaneous reaction typically involves both a decrease in enthalpy and an increase in entropy.
What are non-spontaneous processes and how can they be identified?
-Non-spontaneous processes are those that do not occur naturally without external energy input. They can be identified by an increase in enthalpy (indicating the products have more energy than the reactants) and a decrease in entropy (indicating less dispersal of matter).
What is the equilibrium constant and how does it relate to spontaneity?
-The equilibrium constant (K) is a measure that compares the concentration of products to the concentration of reactants. A K value greater than 1 indicates a spontaneous process favoring the products, while a K value less than 1 indicates a non-spontaneous process favoring the reactants.
How does the video use simulations to demonstrate the concept of spontaneity?
-The video uses PHET simulations to visually represent the movement of molecules from a high-energy, concentrated state (reactants) to a lower-energy, dispersed state (products). The change in the number of molecules on each side and their energy levels help to illustrate the concepts of enthalpy and entropy in the context of spontaneity.
What is the significance of the rusting of iron in the video?
-The rusting of iron is used as an example of a spontaneous reaction. It involves a negative enthalpy change, indicating that the reaction releases energy, and an increase in entropy, as the iron atoms become more dispersed in the form of rust.
How does the video explain the transformation of graphite to diamond?
-The video explains that the transformation of graphite to diamond is a non-spontaneous process. It requires a large amount of energy and pressure, and does not occur naturally without external intervention.
What term is suggested to replace 'spontaneous process' to avoid confusion?
-The video suggests using the term 'thermodynamically favored process' instead of 'spontaneous process' to more accurately describe reactions that naturally favor the formation of products over reactants.
What is the next step for understanding reactions that do not fit neatly into the categories of spontaneous or non-spontaneous?
-The video suggests that further understanding of reactions that do not fit neatly into the categories of spontaneous or non-spontaneous requires learning about Gibbs Free Energy, which will be covered in the next video.
Outlines
📚 Introduction to Spontaneous Processes
This paragraph introduces the concept of spontaneous processes, which are processes that occur without the need for external energy. The video begins with a demonstration using a tower, illustrating that while knocking it over is a spontaneous process, it requires a small amount of energy to initiate. The main theme revolves around the idea that spontaneous processes are characterized by a decrease in enthalpy (internal energy) and an increase in entropy (randomness or dispersal of matter). The paragraph also explains that while enthalpy and internal energy are used interchangeably, there are slight differences between them. It sets the stage for understanding spontaneous versus non-spontaneous reactions and introduces the concept of the equilibrium constant as a tool for predicting whether a process will favor products or reactants.
🔄 Understanding Spontaneity through Entropy and Enthalpy
This paragraph delves deeper into the factors that determine the spontaneity of a process, focusing on the roles of enthalpy and entropy. It explains how a decrease in enthalpy and an increase in entropy are indicative of a spontaneous process, while the opposite would suggest a non-spontaneous reaction. The paragraph uses the example of a PHET simulation to visually demonstrate how changes in energy and molecular distribution can affect the spontaneity of a reaction. It also discusses the equilibrium constant (K value) and how it can be used to predict the favorability of products or reactants. The summary emphasizes the importance of considering both enthalpy and entropy when assessing spontaneity and introduces the concept of Gibbs Free Energy, which will be explored in a subsequent video.
Mindmap
Keywords
💡Spontaneous processes
💡Enthalpy
💡Entropy
💡Equilibrium constant
💡Non-spontaneous reactions
💡Thermodynamically favored process
💡Exothermic reactions
💡Endothermic reactions
💡Gibbs Free Energy
💡Reversible reactions
💡Thermite reaction
Highlights
The introduction of spontaneous processes as processes that occur without external energy. (Start time: 0s)
Demonstration of a spontaneous process using the knocking over of a tower. (Start time: 3s)
Explanation that spontaneous processes move in one direction and are statistically impossible to reverse. (Start time: 7s)
The treatment of enthalpy and internal energy as synonyms for the purpose of understanding spontaneous reactions. (Start time: 12s)
The identification of spontaneous processes by a decrease in enthalpy and an increase in entropy. (Start time: 19s)
The use of the equilibrium constant to determine if a process favors products or reactants. (Start time: 29s)
The model demonstrating how an equilibrium constant greater than 1 favors products, while less than 1 favors reactants. (Start time: 37s)
The discussion on the non-occurrence of spontaneous reactions without external energy, using the example of graphite turning into diamond. (Start time: 44s)
The suggestion to use the term 'thermodynamically favored process' instead of 'spontaneous process' for clarity. (Start time: 49s)
The importance of considering both enthalpy and entropy when determining if a reaction is spontaneous. (Start time: 56s)
The example of a thermite reaction being exothermic and thus a spontaneous reaction due to the energy difference between reactants and products. (Start time: 1m 3s)
The use of a PHET simulation to visually demonstrate the effect of entropy and enthalpy changes on the spontaneity of a reaction. (Start time: 1m 12s)
The explanation that an increase in entropy can be observed without a change in temperature, using the cold pack example. (Start time: 1m 23s)
The method to calculate the equilibrium constant as the concentration of products divided by the concentration of reactants. (Start time: 1m 34s)
The demonstration of how changing the energy of reactants can influence the spontaneity of a reaction using a simulation. (Start time: 1m 46s)
The summary of the conditions for a spontaneous reaction: decrease in enthalpy and increase in entropy, and the conditions for a non-spontaneous reaction: increase in enthalpy and decrease in entropy. (Start time: 2m 18s)
Transcripts
Browse More Related Video
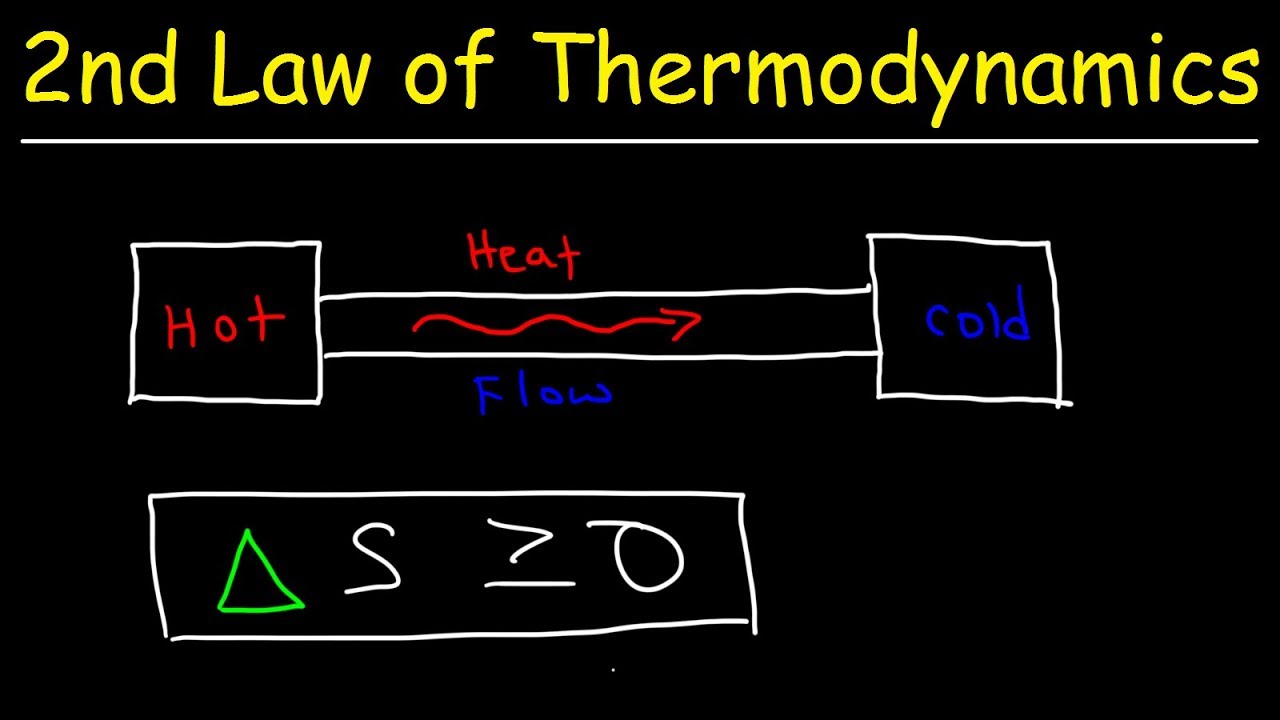
Second Law of Thermodynamics - Heat Energy, Entropy & Spontaneous Processes

Entropy: Embrace the Chaos! Crash Course Chemistry #20

18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemistry

6.2 Entropy, Gibbs Free Energy, and the Equilibrium Constant | Organic Chemistry
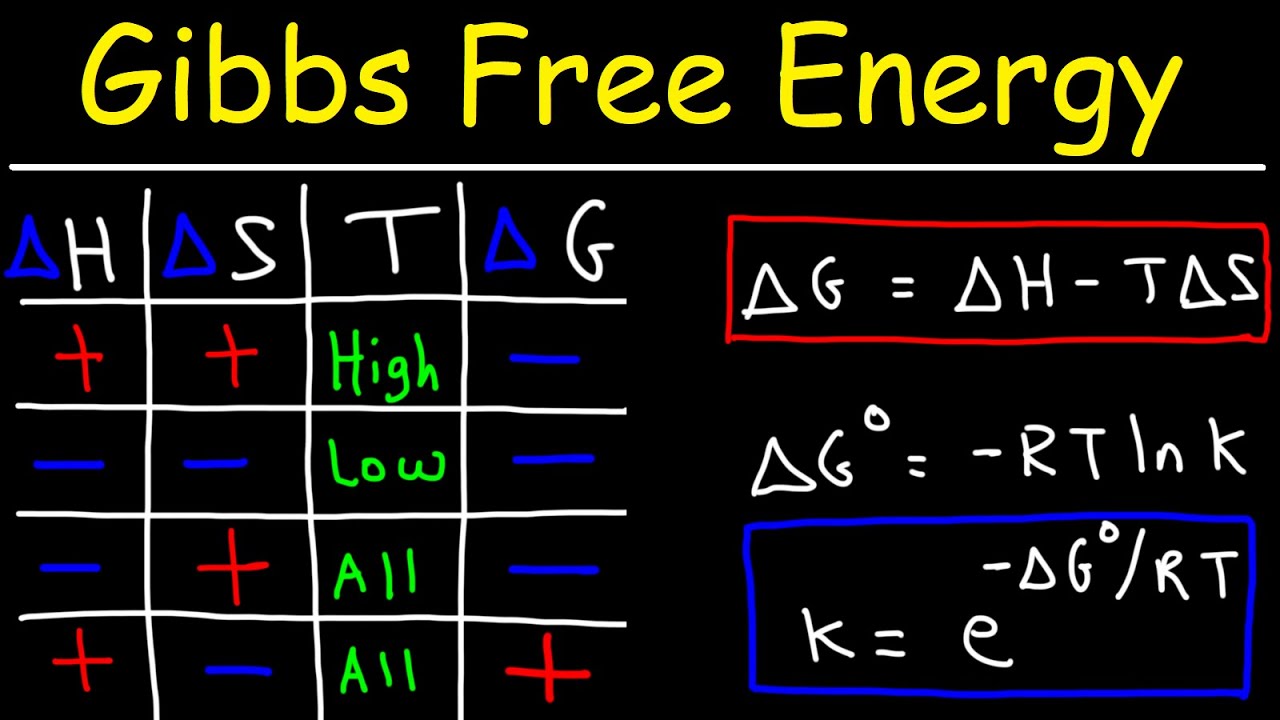
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K

16. Thermodynamics: Gibbs Free Energy and Entropy
5.0 / 5 (0 votes)
Thanks for rating: