ALEKS - Interconverting pH and Hydronium Ion Concentration
TLDRThis educational video discusses the conversion between pH and hydronium ion concentration, emphasizing the importance of significant figures. The instructor demonstrates how to calculate pH from hydronium ion concentration using logarithms and vice versa, highlighting the need to match the number of significant figures in concentration to the decimal places in pH values.
Takeaways
- 📚 The topic is about interconverting pH and hydronium ion concentration, which is a fundamental concept.
- 🔢 Converting from the concentration of H3O+ to pH involves taking the negative logarithm of the concentration.
- 👨🏫 The instructor emphasizes the importance of significant figures and their relation to the decimal places in the pH value.
- ✅ An example calculation is provided: taking the negative log of 6.3 * 10^-7 results in a pH of 6.22, rounded to two decimal places.
- 🔄 To convert from pH to concentration, the inverse process is used, involving 10 raised to the negative power of the pH value.
- 📉 An example of converting 10.14 to concentration results in 7.244 * 10^-11, which is rounded to 7.2 * 10^-11 to match the significant figures.
- 📈 Another example is converting a concentration of 81 to a pH value, resulting in 2.91, which is then rounded to 2.92 to reflect three significant figures.
- 📝 The instructor explains that the number of significant figures in the concentration should equal the number of decimal places in the pH.
- 🧮 The concept of significant figures is linked to the mathematical approach of logarithms and their impact on the pH calculation.
- 🤔 The instructor suggests consulting a math professor for a more in-depth explanation of the relationship between logarithms and significant figures.
- ❓ The instructor invites questions from the class, indicating an openness to further discussion and clarification.
Q & A
What is the basic process for converting hydronium ion concentration to pH?
-The basic process involves taking the negative logarithm (pH = -log[H3O+]) of the hydronium ion concentration.
How is the pH value calculated in the provided example with a hydronium ion concentration of 6.3 * 10^-7?
-The pH is calculated by taking the negative logarithm of 6.3 * 10^-7, which results in a pH of approximately 6.20.
Why is it important to consider significant figures when calculating pH from hydronium ion concentration?
-Significant figures are important to ensure the precision and accuracy of the calculated pH value, reflecting the level of certainty in the measurement.
How many significant figures are there in the hydronium ion concentration of 6.3 * 10^-7?
-There are two significant figures in the given hydronium ion concentration (6.3).
What is the relationship between the number of significant figures and decimal places in pH calculation?
-The number of significant figures in the hydronium ion concentration should equal the number of decimal places in the calculated pH value.
How is the hydronium ion concentration calculated from a given pH value?
-The hydronium ion concentration is calculated by taking 10 raised to the negative power of the pH value (e.g., [H3O+] = 10^-14 for a pH of 14).
In the example, how is the hydronium ion concentration calculated from a pH of 10.14?
-The hydronium ion concentration is calculated as 10^-10.14, which is approximately 7.2 * 10^-11.
What is the significance of the number of decimal places in the hydronium ion concentration calculation?
-The number of decimal places indicates the precision of the concentration value and should match the number of significant figures in the pH value used for the calculation.
How many significant figures are there in the concentration value of 81?
-There are three significant figures in the concentration value of 81 (8, 1, and the trailing zero after the decimal point).
What is the final pH value calculated from a hydronium ion concentration of 81, and why is it rounded to 2.92?
-The pH is calculated as 2.9115 from the negative logarithm of 81. It is rounded to 2.92 to match the three significant figures in the original concentration value.
Outlines
🔢 Converting pH to Hydronium Ion Concentration
This paragraph explains the process of converting between pH and hydronium ion concentration, with a focus on the correct use of significant figures. The lecturer demonstrates how to calculate pH from a given concentration of H3+ ions using the negative logarithm, resulting in a pH of 6.22 with two significant figures. The importance of matching the number of significant figures in the original concentration to the decimal places in the pH value is emphasized. The lecturer also covers the reverse process, converting pH back to hydronium ion concentration, and explains how to determine the correct number of significant figures in the answer.
Mindmap
Keywords
💡pH
💡hydronium ion concentration
💡significant figures
💡logarithm
💡negative log
💡decimal places
💡conversion
💡10 to the minus 10
💡scientific notation
💡rounding
💡mathematical precision
Highlights
Introduction to the topic of interconverting pH and hydronium ion concentration.
Explanation of the basic process for converting from concentration of H3O+ to pH using the negative logarithm.
Demonstration of calculating pH with a given concentration of H3O+ as 6.3 * 10^-7.
Clarification on the significance of significant figures in pH calculation.
Guidance on matching the number of significant figures to decimal places in pH values.
Conversion of pH to hydronium ion concentration using the inverse of the negative log.
Example calculation of hydronium ion concentration from a pH value of 10.14.
Emphasis on determining the correct number of significant figures for the hydronium ion concentration.
Instruction on rounding the hydronium ion concentration to two significant figures.
Process of converting a hydronium ion concentration back to pH using the negative log.
Example calculation of pH from a concentration of 81.
Discussion on the importance of leading zeros in determining significant figures.
Rounding the calculated pH value to match the number of significant figures.
Final pH value presented to Alex with a focus on significant figures.
Recap of the relationship between significant figures in concentration and decimal places in pH.
Advice on ensuring the proper mathematical approach to significant figures in pH calculations.
Encouragement for students to seek further explanation from a math professor if needed.
Closing remarks inviting students to ask further questions about the topic.
Transcripts
Browse More Related Video

How to find the Ka of an acid when given pH

Calculate the pH of Acids and Bases Given the Concentration of a Solution
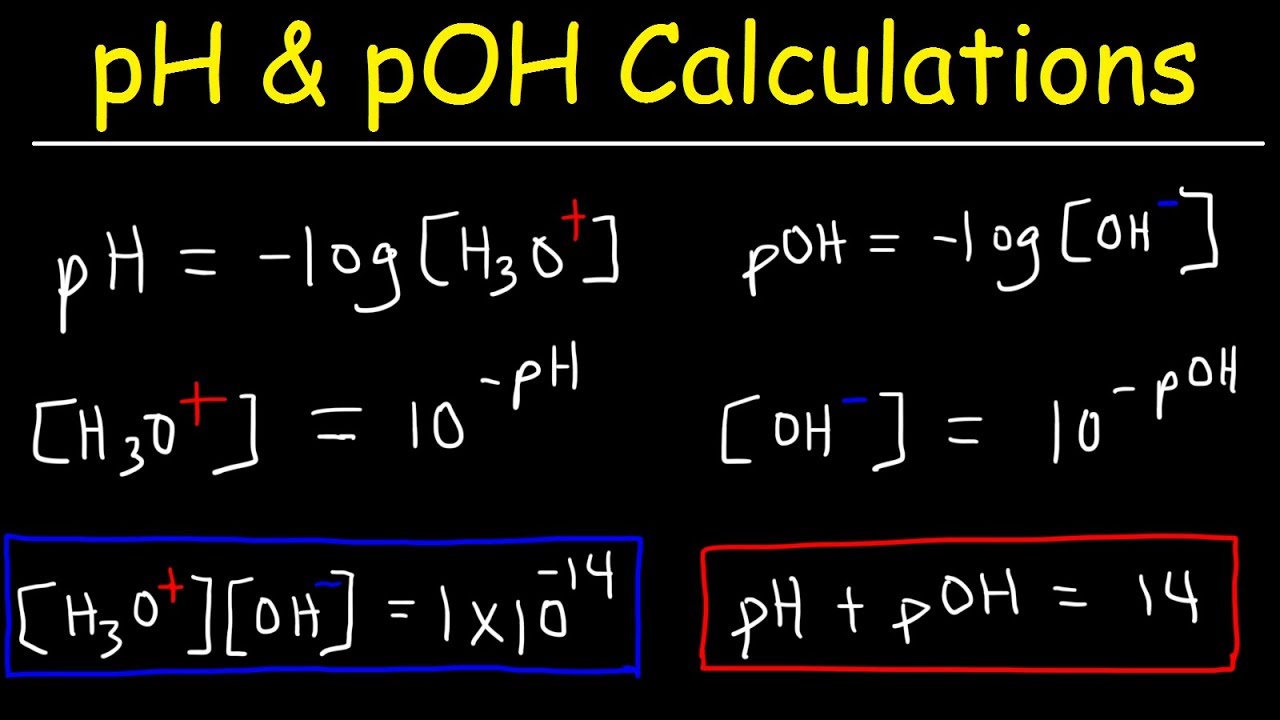
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems
pH and pOH: Crash Course Chemistry #30

Aleks Interconverting Ka and pKa
Introduction to pH, pOH, and pKw
5.0 / 5 (0 votes)
Thanks for rating: