[H2 Chemistry] 2021 Kinetics 1
TLDRThis chemistry lecture delves into reaction kinetics, emphasizing the difference between thermodynamic feasibility and kinetic accessibility of reactions. It explains the concepts of activation energy, rate equations, and reaction orders, highlighting the importance of understanding reaction mechanisms. The instructor discusses various aspects of kinetics, including the determination of rate equations, the impact of concentration and temperature on reaction rates, and the use of graphs to analyze reaction orders. The lecture aims to provide a solid foundation for students to appreciate the nuances of reaction kinetics and its significance in advanced chemistry studies.
Takeaways
- 🔍 The script is a lecture on reaction kinetics, emphasizing the difference between thermodynamics and kinetics in determining whether a reaction will occur and how quickly it will proceed.
- 📉 The importance of enthalpy and entropy changes in assessing the feasibility of a reaction is highlighted, but the focus shifts to the rate of reaction, which can be slow even for thermodynamically favorable reactions.
- 💎 The concept of activation energy as a kinetic barrier is introduced, explaining that a high activation energy can slow down reactions, using the example of diamond transforming into graphite.
- 📚 The lecture outlines the learning objectives for a course on kinetics, including understanding reaction mechanisms and the factors affecting reaction rates.
- 🔁 The difference between the overall balanced equation of a reaction and the detailed reaction mechanism, including intermediate steps, is explained, emphasizing the importance of the latter in understanding the system.
- ⏱️ The rate of reaction is defined mathematically as the change in concentration of reactants or products per unit time, with the negative sign indicating the decrease in concentration of reactants.
- 📉 Different types of reaction rates are discussed, including instantaneous rate, initial rate, and average rate, each providing different insights into the progress of a reaction.
- 🔢 The rate equation is introduced as an experimentally determined relationship between the rate of reaction and the concentrations of reactants, with the order of reaction and the rate constant being key parameters.
- 🌡️ The rate constant is sensitive to temperature and is affected by the presence of a catalyst, which provides an alternative pathway for the reaction, not by lowering the activation energy.
- 📈 The process of determining the rate equation involves plotting different types of graphs, such as concentration against time, rate against concentration, and rate against time, to deduce the order of the reaction.
Q & A
What is the main difference between thermodynamics and kinetics when studying chemical reactions?
-Thermodynamics focuses on the enthalpy and entropy changes to determine if a reaction is feasible or spontaneous, while kinetics deals with the rate at which reactions occur, including the factors that influence the speed of reactions, such as activation energy.
Why is it important to consider both enthalpy and entropy changes when determining if a reaction is thermodynamically feasible?
-Both enthalpy and entropy changes are crucial because they influence the overall Gibbs free energy change (ΔG) of a reaction. A reaction is thermodynamically feasible if ΔG is negative, which can occur even if the reaction is endothermic (ΔH > 0) if there is a sufficiently large increase in entropy (ΔS).
What is an activation barrier, and why is it significant in reaction kinetics?
-An activation barrier, or activation energy, is the minimum energy needed for reactants to collide and form products. It is significant because it determines the rate of a reaction; a higher activation barrier generally results in a slower reaction rate.
Can a thermodynamically feasible reaction be slow or take a long time to occur? Why?
-Yes, a thermodynamically feasible reaction can be slow if it has a high activation barrier. Even though the reaction is energetically favorable, the high activation energy can slow down the rate at which reactants transform into products.
What is the difference between the stoichiometric coefficient in a balanced chemical equation and the order of a reaction?
-The stoichiometric coefficient indicates the proportions in which reactants combine to form products, while the order of a reaction refers to how the rate of the reaction is related to the concentrations of the reactants. The order is determined experimentally and does not necessarily correspond to the stoichiometric coefficients.
Why is the study of reaction mechanisms important in understanding chemical reactions?
-Studying reaction mechanisms is important because it reveals the intermediate steps and the sequence of elementary reactions that occur during the overall reaction. This understanding can help chemists predict the course of reactions and design reactions that meet specific goals.
What does the rate equation represent, and how is it used in kinetics?
-The rate equation represents the relationship between the rate of a reaction and the concentrations of the reactants. It is used to predict how changes in reactant concentrations will affect the reaction rate and to determine the order of the reaction with respect to each reactant.
What are the three types of reaction orders commonly encountered in kinetics?
-The three common types of reaction orders are zero order, first order, and second order. Zero order reactions have a rate independent of the reactant concentration, first order reactions have a rate directly proportional to the concentration of one reactant, and second order reactions have a rate proportional to the square of the concentration of one reactant.
How can you determine the order of a reaction experimentally?
-You can determine the order of a reaction experimentally by measuring how the rate of the reaction changes with changes in the concentration of the reactants. This can involve plotting concentration-time graphs, rate against concentration graphs, or using logarithmic plots to linearize the relationship and extract the order from the slope or intercept.
What is the significance of the rate constant (k) in a rate equation, and how does it relate to temperature?
-The rate constant (k) is a proportionality constant in the rate equation that quantifies the intrinsic rate at which a reaction occurs. It is sensitive to temperature, with higher temperatures generally increasing the rate constant due to the Arrhenius equation, which describes the temperature dependence of reaction rates.
Outlines
🔬 Introduction to Reaction Kinetics and Thermodynamics
This paragraph introduces the topic of reaction kinetics, following a discussion on thermodynamics. It emphasizes the importance of enthalpy and entropy changes in determining whether a reaction is feasible or spontaneous, as described by the equation delta G = delta H - T delta S. The instructor clarifies that while exothermic reactions are often assumed to be spontaneous, entropy contributions are equally significant. The paragraph transitions into the concept of reaction kinetics, highlighting that a thermodynamically feasible reaction might be slow, using the example of diamond transforming into graphite. The activation energy, or kinetic barrier, is introduced as a key factor in reaction rates, and the instructor mentions that reaction kinetics will be explored in more detail, including rate equations and reaction orders.
🧪 The Importance of Studying Reaction Kinetics
The instructor underscores the significance of studying reaction kinetics for understanding the mechanisms by which reactions proceed. They explain that while chemical equations provide information about reactants and products, they do not reveal the intermediate steps or the 'what happens in between.' The paragraph delves into the curiosity of chemists to understand these intermediate steps, which can inform the design of reactions to achieve specific outcomes. The concept of reaction mechanisms is introduced as a fundamental aspect of study in organic chemistry, with five basic mechanisms to be aware of. The instructor encourages students to build a strong foundation in kinetics to ease their studies in later years, acknowledging that initial understanding may be shallow but will deepen over time.
📚 Defining and Calculating Reaction Rates
This paragraph focuses on defining the rate of a chemical reaction, which is the change in concentration of a reactant or product per unit time. The instructor discusses the mathematical representation of reaction rates, emphasizing that the rate of disappearance of reactants is indicated by a negative slope due to decreasing concentration over time. However, calculated rates are positive numbers. The paragraph also addresses common misconceptions regarding rate units, explaining that they should reflect the change in concentration (e.g., moles per liter per second or per minute). An example of a simple reaction between H2 and I2 to form HI is used to illustrate how the rates of disappearance of reactants and the rate of product formation are related, highlighting the need to adjust for stoichiometric ratios.
📉 Understanding Instantaneous and Average Rates
The instructor introduces different types of reaction rates, particularly instantaneous and average rates. Instantaneous rate is likened to taking the derivative at a specific time on a concentration-time graph, reflecting the rate at that exact moment. Initial rate is the instantaneous rate at time equals zero, which is crucial for understanding how fast a reaction begins. Average rate is defined over a time interval, represented by a straight line on a graph, indicating the overall change in concentration over that period. The paragraph clarifies the mathematical concepts behind these rates, using graphs to illustrate the differences and emphasizing the importance of understanding the mathematical principles for studying chemistry.
🔍 Analyzing Data to Determine Reaction Rates
This paragraph discusses a practical approach to determining reaction rates using data from a graph of a reaction between CH3Br and OH-. The reaction is identified as a nucleophilic substitution, where OH- acts as a nucleophile. The instructor guides students on how to analyze the graph to find the initial rates by drawing a tangent at time equals zero and explains the significance of the units for the rates. The paragraph also touches on the process of determining the instantaneous rate at a specific time and the average rate over a given period, highlighting the importance of careful data interpretation and graph analysis in understanding reaction kinetics.
🌐 The Concept of Rate Equations, Orders, and Rate Constants
The instructor introduces the technical aspects of rate equations, emphasizing that the order of reaction is not the same as the stoichiometric coefficient. The rate equation is presented as a product of the rate constant (k) and the concentrations of reactants raised to the power of their respective orders. The paragraph explains that the order of reaction must be determined experimentally and is not necessarily related to the stoichiometric coefficients. The rate constant is described as a proportionality constant that is sensitive to temperature and is also determined experimentally. The instructor warns against common misconceptions and stresses the importance of understanding the fundamental definitions and concepts before progressing to more complex topics.
🔧 Experimentation and the Determination of Reaction Orders
This paragraph delves into the experimental determination of reaction orders, explaining that experiments are designed to test how changes in concentration affect reaction rates. The instructor introduces the concepts of zero-order, first-order, and second-order reactions, describing their characteristics and how they relate to the concentration of reactants. Zero-order reactions show rate independence from concentration, first-order reactions have a direct proportionality to the concentration, and second-order reactions show a rate proportional to the square of the concentration. The paragraph emphasizes the importance of understanding these orders through detailed study and practical exposure to different scenarios.
📚 Writing Rate Equations and Understanding Rate Constants
The instructor provides a clear example of writing a rate equation for a reaction between hydrogen peroxide and acidified iodine ions, given the orders with respect to H2O2 and H+ are first and zero, respectively. The rate equation is simplified, and the overall order of the reaction is determined. The paragraph also discusses the rate constant, explaining its dependence on temperature and presence of a catalyst, and clarifies misconceptions about catalysts and activation barriers. The rate constant's units are explored, demonstrating how they relate to the overall order of the reaction.
📈 Determining Rate Equations Through Graphs
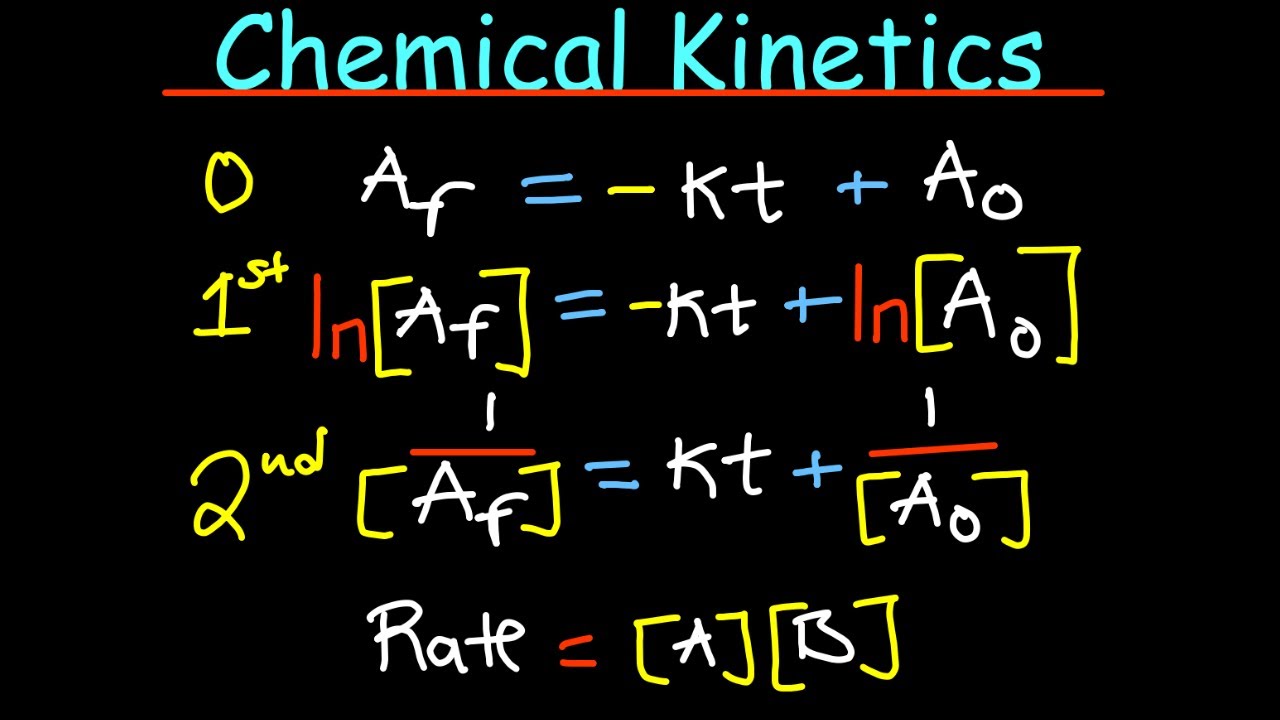
This paragraph outlines the process of determining rate equations using different types of graphs: concentration-time, rate against concentration, and rate against time. The instructor emphasizes the importance of understanding the shapes of these graphs and their mathematical implications for determining the order of reactions. The paragraph provides examples of how to interpret concentration-time graphs for zero-order, first-order, and second-order reactions, highlighting the significance of half-life and its relationship to reaction order. The instructor advises against memorization and instead encourages a mathematical understanding of the concepts.
📉 Analyzing Graphs to Understand Reaction Kinetics
The instructor continues the discussion on graph analysis for understanding reaction kinetics, focusing on rate against concentration graphs. For zero-order reactions, a horizontal line is expected, while first-order reactions yield a straight line passing through the origin. The paragraph introduces the technique of linearizing graphs by plotting log rates against log concentration to determine the order of reaction and the rate constant. The method is explained as a valuable tool when the reaction order is unknown. The instructor also briefly mentions rate against time graphs, noting their less intuitive nature and suggesting that understanding the mathematics behind the graphs is more important than memorizing their shapes.
Mindmap
Keywords
💡Thermodynamics
💡Reaction Kinetics
💡Activation Energy
💡Reaction Mechanism
💡Rate Equation
💡Stoichiometric Coefficient
💡Rate Constant
💡Order of Reaction
💡Instantaneous Rate
💡Half-Life
Highlights
Reaction kinetics involves understanding the rate at which reactions occur and the factors that influence these rates.
Thermodynamics and kinetics are both essential for determining whether a reaction is feasible and the speed at which it will occur.
The concept of activation energy as a barrier to the reaction, highlighting its importance in reaction kinetics.
The distinction between enthalpy and entropy changes in reactions and their impact on the feasibility of a reaction.
The study of reaction mechanisms to understand the intermediate steps in a chemical reaction.
The definition of rate equations and the significance of order and rate constants in kinetics.
The importance of recognizing that the stoichiometric coefficient is not equivalent to the order of reaction.
The impact of temperature on reaction rates and the role of the Arrhenius equation in understanding this relationship.
The process of determining the order of a reaction through experimental data and graphical analysis.
The use of half-life in zero, first, and second-order reactions to determine reaction rates and constants.
The significance of understanding the difference between instantaneous rate, initial rate, and average rate.
The method of linearizing graphs to determine the order of reaction and the rate constant from experimental data.
The practical applications of kinetics in organic chemistry and the importance of foundational knowledge for further studies.
The role of catalysts in providing alternative pathways for reactions without lowering the activation barrier.
The mathematical approach to understanding and determining the units for rate constants in different order reactions.
The comparison between thermodynamics and kinetics in terms of experimental procedures and student engagement.
The importance of not memorizing the shapes of graphs but understanding the mathematical principles behind them in kinetics.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: