Chemical Kinetics Tutorial Sheet 2024 MUL
TLDRThis chemistry tutorial video script covers a comprehensive session on chemical kinetics, addressing various questions related to reaction rates, orders, and mechanisms. The instructor guides viewers through the process of calculating average rates, determining reaction orders using initial rates and concentration changes, and elucidating reaction mechanisms. The script also delves into finding rate constants and explores specific reaction scenarios, such as the decomposition of H2O2 and the oxidation of bromide ions, providing step-by-step solutions and explanations.
Takeaways
- 📚 The script covers a chemistry kinetics tutorial, focusing on problem-solving for various questions related to chemical reaction rates and orders.
- 🔍 The tutorial explains how to calculate average rates of decomposition and formation for chemical reactions, emphasizing the importance of stoichiometric coefficients in determining the rate expressions.
- 🧪 The concept of reaction orders is explored, detailing how to determine the order of a reaction with respect to different reactants using given data and initial rates.
- 📉 The script discusses the process of identifying reaction mechanisms and determining the rate-determining step, which is crucial for understanding the overall rate law of a reaction.
- 📝 It illustrates the use of integrated rate laws, particularly for first-order reactions, to find the percentage of a reactant that decomposes over time.
- ⚗️ The tutorial provides step-by-step solutions for calculating rate constants and elucidates the significance of these constants in rate laws.
- 🔢 The importance of units in rate laws is highlighted, showing how to derive the units of the rate constant from the overall order of the reaction.
- 📐 The script introduces the method of comparing experiments where two of the reactants are held constant to solve for the reaction order with respect to the varying reactant.
- 🔄 The concept of intermediates in reaction mechanisms is discussed, and their role in determining the rate-determining step is explained.
- 📊 Data from experiments is used to demonstrate how to apply the initial rate method to deduce the rate law and rate constant for a reaction.
- 🔑 The script concludes with a summary of the key learnings and provides access to further resources for in-depth learning and additional practice problems.
Q & A
What is the formula to calculate the average rate of decomposition of a reactant in a chemical reaction?
-The formula to calculate the average rate of decomposition is given by the negative change in concentration of the reactant divided by the time taken for the reaction, multiplied by the stoichiometric coefficient of the reactant in the balanced chemical equation.
How does the stoichiometric coefficient affect the rate of a chemical reaction?
-The stoichiometric coefficient determines the amount of each reactant and product involved in a balanced chemical reaction. For the rate of a reaction concerning a reactant, the rate is inversely proportional to the stoichiometric coefficient of that reactant.
What is the significance of the rate constant (K) in a reaction rate expression?
-The rate constant (K) in a reaction rate expression indicates the proportionality between the rate of the reaction and the concentrations of the reactants raised to their respective reaction orders. It is a measure of the reaction's intrinsic speed and is specific to the reaction and conditions under which it is measured.
How can you determine the reaction order with respect to a particular reactant?
-To determine the reaction order with respect to a particular reactant, you can compare experiments where the concentrations of all other reactants are held constant while the concentration of the reactant in question is varied. The ratio of the rates for these experiments will give you the reaction order for that reactant.
What does it mean if a reactant has a reaction order of zero in a rate law?
-If a reactant has a reaction order of zero in a rate law, it implies that the concentration of that reactant does not affect the rate of the reaction. This is often the case for intermediates or catalysts that are present in sufficient quantities to not limit the reaction rate.
How can you find the rate constant for a reaction given its rate law and initial conditions?
-To find the rate constant for a reaction, you can use the rate law and substitute the known initial concentrations of the reactants along with the initial rate of the reaction into the rate law equation. Solving for K will give you the rate constant.
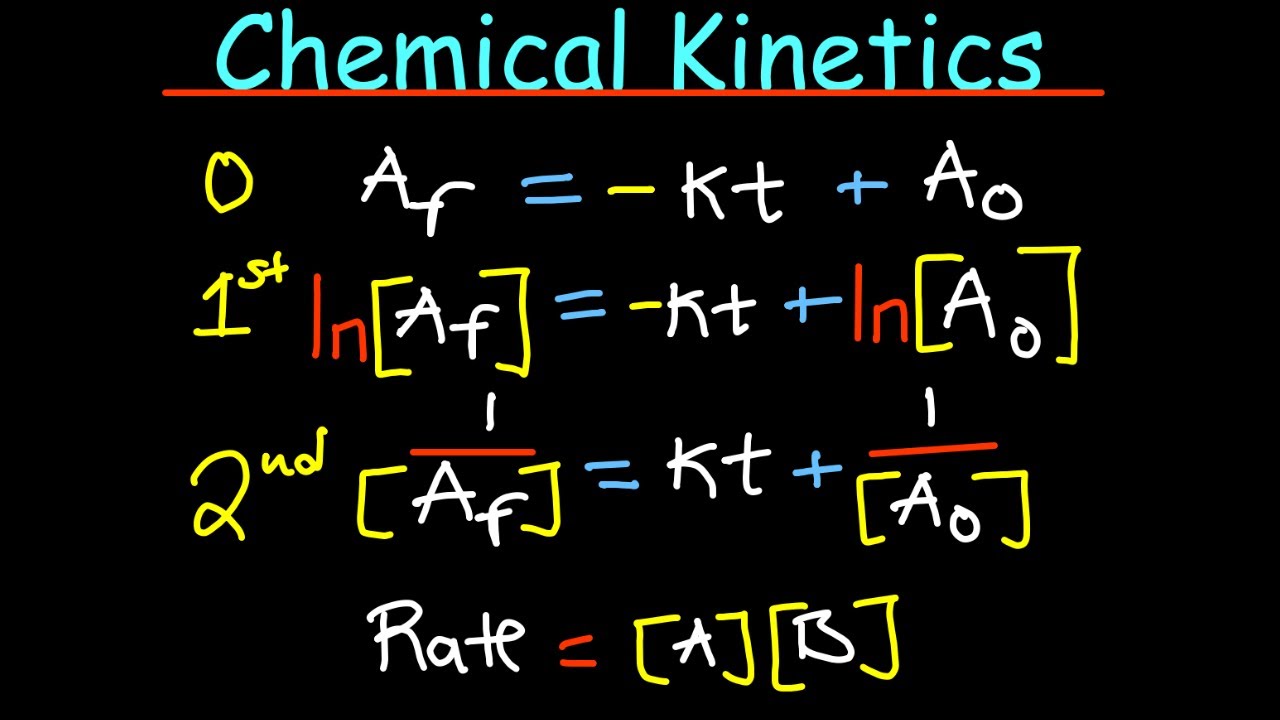
What is the integrated rate law for a first-order reaction?
-The integrated rate law for a first-order reaction is given by ln([A]t / [A]0) = -kt, where [A]t is the concentration of reactant A at time t, [A]0 is the initial concentration of A, k is the rate constant, and t is the time.
How can you determine the percentage of a reactant that has decomposed over time?
-To determine the percentage of a reactant that has decomposed, you can use the ratio of the final concentration to the initial concentration found from the integrated rate law. The percentage decomposed is 100% minus the percentage that remains.
What is the relationship between the rate of formation and the rate of consumption for reactants and products in a reaction?
-For a balanced chemical reaction, the rate of formation of products is equal to the rate of consumption of reactants. If the stoichiometry of a product is twice that of a reactant, then the rate of formation of the product is twice the rate of consumption of the reactant.
How can you determine if a substance is acting as a catalyst in a reaction mechanism?
-A substance acts as a catalyst in a reaction mechanism if it is not consumed in the overall reaction and it increases the rate of the reaction without being part of the final product. If its concentration increases the rate decreases, it is not a catalyst but may be an inhibitor.
Outlines
📚 Chemical Kinetics Tutorial Overview
The video script introduces a tutorial session on chemical kinetics, marking it as the fourth in the series. The presenter outlines the numerous questions to be addressed, including determining the rate of decomposition, reaction orders, and understanding reaction mechanisms. Specific focus is given to the rate of decomposition of a reactant, emphasizing the importance of considering stoichiometric coefficients and the change in concentration over time. The tutorial promises to delve into each question methodically, with a link provided in the description for access to the full video content.
🧪 Calculating Average Rate of Reaction
This paragraph delves into the calculation of the average rate of decomposition for a chemical reaction. The process involves understanding the stoichiometry of the reaction and applying the formula for rate, which accounts for the change in concentration over a given time period. The example provided walks through the calculation steps, including determining the final and initial concentrations and the time interval. The result is a rate value expressed in moles per second, highlighting the importance of ensuring the rate is positive by incorporating the stoichiometric coefficient.
🔍 Determining Reaction Orders from Experimental Data
The focus shifts to analyzing experimental data to determine the reaction orders with respect to different reactants. The method involves comparing experiments where two of the reactants are held constant while the third varies, allowing for the calculation of the reaction order for each reactant. The 'rate law' is introduced as a formula that relates the rate of reaction to the concentrations of the reactants raised to certain powers. The paragraph illustrates the step-by-step process of isolating the reaction order for each reactant and emphasizes the importance of identifying experiments where conditions align for such calculations.
🧐 Analyzing Reaction Mechanisms and Rates
The script discusses the analysis of a chemical reaction mechanism and its consistency with the observed rate law. It involves breaking down the reaction into elementary steps and identifying the rate-determining step, which is crucial for understanding the overall reaction rate. The paragraph explains how to derive expressions for the intermediate species and how these expressions can be substituted into the rate equation to match the experimentally determined rate law. The process requires a keen eye for detail and a strong grasp of stoichiometry and reaction kinetics.
🔍 Further Exploration of Reaction Orders and Rate Constants
Continuing from the previous discussion, this paragraph further explores the determination of reaction orders and rate constants using experimental data. It emphasizes the methodical approach of comparing different experiments to isolate the reaction orders of individual reactants. The paragraph also explains how to calculate the rate constant by substituting known values into the rate equation. The importance of understanding the overall order of the reaction and the units of the rate constant is highlighted, with a practical example provided for clarity.
📉 Integrated Rate Law and Reaction Analysis
This section of the script introduces the concept of the integrated rate law, particularly focusing on first-order reactions. It explains how to use the integrated rate law to calculate the percentage decomposition of a reactant over time, using the natural logarithm and exponential functions. The paragraph provides a step-by-step guide on manipulating the integrated rate law to find the ratio of final to initial concentration, which can then be used to determine the percentage of reactant decomposed. The explanation is practical, offering a clear method for solving such problems in chemical kinetics.
📝 Initial Rate Method Application
The script discusses the application of the initial rate method to the decomposition of nitrogen dioxide, providing experimental data for two different experiments. The goal is to find the rate law and the rate constant with respect to the formation of oxygen. The paragraph outlines the process of using the initial rate method, which involves comparing the rates and concentrations from different experiments to deduce the reaction orders and calculate the rate constant. The explanation is detailed, ensuring a comprehensive understanding of the method and its application.
🔍 Determining Reaction Orders from Initial Rates
This paragraph focuses on determining the reaction orders for a given reaction using the initial rates from experiments. It explains how to analyze the changes in concentration and rate to deduce the reaction orders for each reactant species. The paragraph provides a clear explanation of how to calculate the overall order of the reaction by summing the individual reaction orders. The importance of understanding the relationship between reactant concentrations and reaction rates is emphasized, with a practical approach to solving such problems in chemical kinetics.
🌐 Conclusion and Further Learning Opportunities
The final paragraph of the script wraps up the tutorial by summarizing the questions addressed and inviting viewers to access further learning materials. It highlights the availability of detailed solutions to the questions discussed in the tutorial on the provided website and Patreon page. The paragraph encourages viewers to register for more in-depth learning and to explore additional resources for a comprehensive understanding of chemical kinetics.
Mindmap
Keywords
💡Chemical Kinetics
💡Reaction Rate
💡Stoichiometric Coefficient
💡Reaction Order
💡Rate Law
💡Catalyst
💡Integrated Rate Law
💡Differential Method
💡Concentration
💡Reaction Mechanism
Highlights
The tutorial covers a range of chemical kinetics problems, including determining reaction orders and rates from given data.
The concept of average rate of decomposition is introduced, explaining how to calculate it using changes in concentration over time.
The tutorial demonstrates how to find the rate of a reaction involving iodide ion oxidation by hypochlorite ion in a basic solution.
The method to determine reaction orders from experimental data by comparing rates while keeping other reactants constant is explained.
The importance of the stoichiometric coefficient in calculating reaction rates is highlighted to ensure positive rate values.
The process of identifying reaction mechanisms and determining the rate law from given reaction steps is discussed.
The tutorial explains how to calculate the rate constant for a reaction using the rate law and experimental data.
The concept of reaction orders with respect to different reactants and how to find them using the rate law is covered.
The tutorial shows how to determine the overall order of a reaction by summing the individual reaction orders.
The method for finding the percentage of a reactant that decomposes over time using the first-order integrated rate equation is demonstrated.
The tutorial addresses how to relate the rate of formation of a product to the rate of reaction of a reactant in a chemical reaction.
The concept of using initial rates to determine the rate law and rate constant for a reaction is explained.
The tutorial provides examples of calculating the rate constant using different experiments and averaging the values.
The method to determine the reaction orders for a decomposition reaction using initial concentration and rate data is shown.
The tutorial explains the use of the integrated rate equation to find the percentage of a reactant that has decomposed at different times.
The process of identifying the rate-determining step in a reaction mechanism is discussed, using the rate law and reaction intermediates.
The tutorial clarifies the role of hydroxide in a reaction, explaining why it is not acting as a catalyst due to its inverse relationship with the rate.
The method for determining the reaction orders and rate constant for a reaction involving nitrogen dioxide and hydrogen is demonstrated.
The tutorial concludes with a discussion on how to apply the rate law to various experiments to find the rate constant and understand reaction kinetics.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: