2022 Live Review 2 | AP Chemistry | Kinetics Multiple-Choice and Free-Response Questions
TLDRThe video script is a comprehensive lecture on the topic of kinetics for AP Chemistry, delivered by Dr. Cacciatore from Charlestown High School in Boston. It covers a range of fundamental concepts including the effects of reaction conditions such as temperature, particle size, and concentration on reaction rates. The lecture delves into interpreting and writing rate laws, calculating rate constants, and understanding concentration-time relationships through integrated rate law equations. It also explores the concept of half-life, collision theory, and reaction mechanisms, and concludes with practice problems to reinforce the concepts taught. The session emphasizes the importance of understanding how these factors influence reaction rates and provides strategies for solving multiple-choice and free-response questions related to kinetics.
Takeaways
- 🎓 **Understanding Kinetics**: Kinetics is a crucial topic for AP Chemistry, covering reaction rates, rate laws, and factors affecting these rates.
- 🔍 **Describing Reaction Effects**: Key factors influencing reaction rates include temperature, particle size, and concentration.
- ✍️ **Writing Rate Laws**: Students learn to interpret and write rate laws, which are essential for calculating the rate constant (k).
- 📚 **Integrated Rate Law**: The integrated rate law equations are used to establish relationships between concentration and time.
- ⏳ **Half-Life Concept**: Understanding half-life (T1/2) is vital, particularly in the context of first-order reactions.
- 💥 **Collision Theory**: This theory helps explain changes in reaction rates at a particle level, focusing on collision frequency, energy, and orientation.
- 📈 **Kinetics Graphs**: Interpreting kinetics graphs is important for connecting reaction parameters and understanding reaction mechanisms.
- 🧩 **Reaction Mechanisms**: Students analyze reaction mechanisms and determine how they align with experimentally determined rate laws.
- 🔢 **Order of Reaction**: Determining the order of a reaction with respect to a specific reactant involves comparing changes in concentration and rate.
- 🌡️ **Temperature's Role**: An increase in temperature generally increases the rate of a chemical reaction due to more frequent and energetic collisions.
- ⚖️ **Entropy Change**: Predicting the sign of the entropy change in a reaction can be deduced from the change in the number of moles of gas involved.
Q & A
What is the main topic covered in the provided transcript?
-The main topic covered in the transcript is kinetics, specifically for AP Chemistry. It includes various aspects such as reaction rates, rate laws, half-life, collision theory, and reaction mechanisms.
What is the role of temperature in affecting the rate of a chemical reaction?
-Temperature affects the rate of a chemical reaction by influencing the average kinetic energy of the particles. Higher temperatures result in more frequent and more forceful collisions, leading to a higher proportion of successful collisions and thus an increased reaction rate.
How can the order of a reaction with respect to a particular reactant be determined?
-The order of a reaction with respect to a particular reactant can be determined by comparing the changes in the initial concentration of that reactant between two experiments while keeping the other reactant concentrations constant. If the rate changes proportionally with the concentration, the reactant is said to be of the first order in the reaction.
What is the significance of the rate constant (k) in the context of rate laws?
-The rate constant (k) is a proportionality constant in the rate law that indicates the intrinsic rate of the reaction at a given temperature. It is specific to the reaction and temperature and is used to calculate the rate of the reaction when the concentrations of the reactants are known.
What is an integrated rate law, and how is it used?
-An integrated rate law is an equation that relates the concentration of a reactant to time for different orders of reactions. It is used to determine the concentration of reactants at a specific time or to calculate the time required for a reactant's concentration to reach a certain level.
How does collision theory explain the effect of particle size on reaction rates?
-According to collision theory, larger particles have a smaller surface area to volume ratio, leading to fewer collisions and thus a lower reaction rate. Smaller particles, on the other hand, have a larger surface area to volume ratio, resulting in more frequent collisions and a higher reaction rate.
What is the relationship between the half-life of a reaction and its rate constant?
-The relationship between the half-life (T1/2) of a reaction and its rate constant (k) is given by the equation k = ln(2) / T1/2. This relationship is particularly useful for first-order reactions.
What is the purpose of the optional handout mentioned in the transcript?
-The optional handout contains all the questions that are discussed during the session. It is provided to help students follow along and review the material more effectively, especially if they find it helpful to have a written reference.
How does the transcript help students prepare for the AP Chemistry exam?
-The transcript guides students through the kinetics topic, which is important for the AP Chemistry exam. It provides explanations, practice questions, and detailed answers that help students understand key concepts, practice problem-solving, and learn how to approach different types of questions.
What are the three factors that affect the rate of a chemical reaction according to collision theory?
-The three factors that affect the rate of a chemical reaction according to collision theory are the frequency of collisions of reactant particles, the kinetic energy of collisions, and the orientation of reactant particles during collisions.
What is the significance of the activation energy in a reaction?
-Activation energy is the minimum amount of energy required for a reaction to occur. It does not change with temperature, but higher temperatures provide particles with more energy, increasing the likelihood of successful collisions and thus the rate of the reaction.
Outlines
👋 Introduction to Kinetics for AP Chemistry
Dr. Cacciatore introduces the topic of kinetics, emphasizing its importance for the AP Chemistry exam. The session will cover the effects of reaction conditions, rate laws, rate constants, integrated rate law equations, half-life concepts, collision theory, and reaction mechanisms. An optional handout is available for additional practice.
🔍 Analyzing Reaction Rates and Particle Size
The script discusses how temperature and particle size affect reaction rates. It guides viewers through multiple-choice questions, illustrating the process of elimination and use of topic numbers for further review. The importance of understanding rate laws and the impact of concentration on reaction time is highlighted.
🧮 Calculating Rate Constants and Half-Life
The video provides a method for calculating the rate constant using the relationship between rate constant and half-life. It also explains how to estimate answers in the absence of a calculator during the AP exam. An example of calculating the fraction of a sample remaining after a certain half-life period is given.
📈 Identifying Zero-Order Reactions and Rate Laws
The script moves on to identifying zero-order reactions using graphs and understanding rate laws. It emphasizes the importance of memorizing the three kinetics graphs and using process of elimination to answer multiple-choice questions correctly.
💡 Collision Theory and Reaction Mechanisms
The role of collision theory in chemical reactions is explained, focusing on how particles collide with sufficient energy for a reaction to occur. The factors affecting the rate of a chemical reaction are discussed, and the concept of reaction mechanisms is introduced, including how to determine the slow step in a reaction.
🧪 Free Response Questions on Reaction Rates
The script presents a long free response question about an experiment involving the decomposition of sodium thiosulfate. It guides students through identifying dependent variables, determining the order of the reaction, calculating the rate constant, and using the integrated rate law to find the time for concentration changes. The importance of graph interpretation and understanding the effect of temperature on reaction rates is also covered.
🌡️ Temperature Effects on Reaction Rates
The video explains how higher temperatures result in increased reaction rates due to more frequent and forceful collisions between particles. It connects this to collision theory and emphasizes the need to answer all questions on the AP exam, even when a calculator is not allowed.
🔍 Determining Reaction Orders and Rate Laws
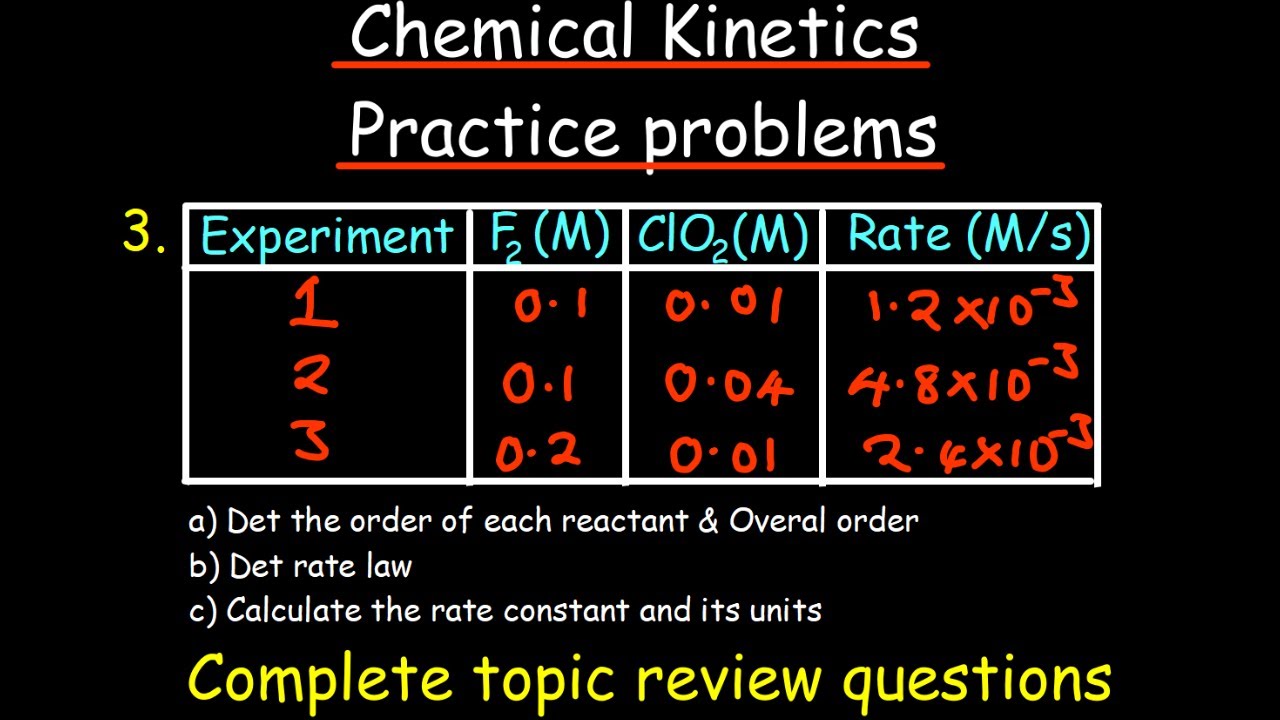
The script guides students through determining the order of a reaction with respect to a specific reactant by comparing trials where only the concentration of that reactant changes. It also covers writing the overall rate law for a reaction and calculating the rate constant from given data.
📝 Balancing Reactions and Identifying Intermediates
The video discusses how to write the balanced equation for an overall reaction from a two-step mechanism and how to identify intermediates and catalysts within a reaction process. It also touches on predicting the sign of entropy change in reactions, linking it to the increase in moles of gas.
📚 Conclusion and Additional Resources
Dr. Cacciatore concludes the session by summarizing key points from the video, encouraging students to utilize additional resources such as AP Daily live review videos. The emphasis is on the comprehensive nature of AP Chemistry, covering multiple units or chapters in exam questions.
Mindmap
Keywords
💡Kinetics
💡Rate Laws
💡Half-Life
💡Collision Theory
💡Reaction Mechanisms
💡Integrated Rate Law
💡Activation Energy
💡Catalyst
💡Enthalpy
💡Entropy
💡Order of Reaction
Highlights
Dr. Cacciatore introduces the topic of kinetics, emphasizing its importance for AP Chemistry exam preparation.
The session covers various factors affecting reaction rates, including temperature, particle size, and concentration.
Students learn to interpret and write rate laws, and calculate the specific rate constant (k).
The use of integrated rate law equations for concentration and time relationships is explained.
Half-life (T1/2) concepts are discussed in the context of first-order reactions.
Collision Theory is used to explain changes in reaction rates at the particle level.
The connection between kinetics graphs and reaction parameters is explored.
An optional handout with questions is available for students to follow along.
Practice multiple-choice questions are used to reinforce learning, with a focus on identifying the slowest reaction rate conditions.
The relationship between temperature and reaction rate is discussed, with a focus on how lower temperatures result in slower reaction rates.
The concept of rate constant (k) is introduced, with an example of calculating it using the half-life of a first-order reaction.
The use of estimation and simplification techniques for solving multiple-choice questions without a calculator is demonstrated.
A problem-solving approach for determining the fraction of a sample remaining after a given time is shown, using the half-life concept.
Zero-order reaction rate evidence is identified through graphical analysis, emphasizing the linear decrease of reactant concentration over time.
Factors influencing the rate of a chemical reaction, as per Collision Theory, are discussed, highlighting the importance of frequency, kinetic energy, and orientation of particle collisions.
The effect of temperature increase on the rate of a chemical reaction is explained through the lens of Collision Theory, focusing on increased collision frequency and energy.
The role of the slow step (rate-determining step) in a reaction mechanism is clarified, showing its impact on the overall reaction rate.
The concept of reaction energy profiles and reaction mechanisms are linked to understand the sequence of energy changes during a reaction.
Free-response questions are tackled, focusing on experimental data analysis, rate law derivation, and rate constant calculation.
The impact of temperature on the observed rate of reaction is explained using particle-level reasoning from Collision Theory.
A step-by-step approach to solving complex free-response questions is provided, encouraging students to persist through each part of the question.
The transcript concludes with a reminder of the综合性 (comprehensive nature) of AP Chemistry free-response questions, which may require knowledge from various chapters.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: