Vapor Pressure - Normal Boiling Point & Clausius Clapeyron Equation
TLDRThis video script delves into the concept of vapor pressure, using water as an example to illustrate how it changes with temperature. It explains the equilibrium between evaporation and condensation, leading to a specific vapor pressure at a given temperature. The script also discusses the Clausius-Clapeyron equation, which relates vapor pressure to temperature, and how elevation affects boiling points, showing that higher elevations decrease the boiling point due to lower atmospheric pressure.
Takeaways
- 💧 Vapor pressure is the equilibrium pressure at which the rate of evaporation equals the rate of condensation of a liquid.
- 🌡️ The rate of evaporation is dependent on the temperature, and it increases as the temperature rises.
- 🔄 Partial pressure can vary widely, but vapor pressure is a specific value at a given temperature where evaporation and condensation are in balance.
- 📈 Vapor pressure increases as temperature increases, but the relationship is not linear; it accelerates at higher temperatures.
- 🌡️➡️🌪️ At 100 degrees Celsius, the vapor pressure of water equals atmospheric pressure (760 torr), which is the definition of the normal boiling point.
- 📉 Elevation affects atmospheric pressure and thus the boiling point of water; higher elevations have lower boiling points.
- 🏞️ In valleys with higher atmospheric pressure, the boiling point of water is higher than at sea level.
- 📚 The Clausius-Clapeyron equation relates vapor pressure and temperature, allowing for the calculation of vapor pressure at different temperatures.
- 🌍 Atmospheric pressure decreases with elevation, which inversely affects the boiling point of substances.
- 🔍 The script provides specific vapor pressure values for water at various temperatures, illustrating the non-linear relationship.
- 🔗 For a deeper understanding, including the full Clausius-Clapeyron formula, viewers are directed to the video's description for more information.
Q & A
What is vapor pressure?
-Vapor pressure is the equilibrium pressure of a vapor in contact with its liquid in a closed system. It is the partial pressure at which the rate of evaporation equals the rate of condensation.
How does the rate of evaporation change with temperature?
-The rate of evaporation increases with temperature. At higher temperatures, more water molecules gain enough energy to transition into the vapor phase.
What is the relationship between the number of water molecules in the air and the rate of condensation?
-The rate of condensation is dependent on the number of water molecules in the air. The more molecules in the vapor phase, the greater the rate of condensation will be.
What is the significance of 23.8 torr in the context of vapor pressure?
-At 23.8 torr, the rate of evaporation equals the rate of condensation at 25 degrees Celsius, making it the vapor pressure of water at that temperature.
What is the normal boiling point of water?
-The normal boiling point of water is 100 degrees Celsius at sea level, where the vapor pressure of water equals the atmospheric pressure of 760 torr.
How does elevation affect the boiling point of water?
-As elevation increases, atmospheric pressure decreases, which in turn lowers the boiling point of water. Conversely, in lower elevations like valleys, the boiling point is higher.
What is the Clausius-Clapeyron equation and how is it used?
-The Clausius-Clapeyron equation relates the vapor pressure and temperature of a substance. It is used to calculate the vapor pressure at different temperatures given the enthalpy of vaporization and the ideal gas constant.
How does the atmospheric pressure change with elevation?
-Atmospheric pressure decreases with increasing elevation. This is because the Earth's atmosphere becomes less dense at higher altitudes.
What is the relationship between the partial pressure of water and its vapor pressure?
-The partial pressure of water can vary and includes values like zero, 15 torr, or 23.8 torr. Vapor pressure, however, is a specific value of the partial pressure where the rates of evaporation and condensation are equal.
Why is it easier to boil water at higher elevations?
-At higher elevations, the atmospheric pressure is lower, which means the boiling point of water is also lower. This requires less energy to reach the boiling point.
How does the rate of condensation change with the pressure of the vapor phase?
-The rate of condensation increases with the pressure in the vapor phase. More molecules in the vapor phase lead to a higher chance of molecules colliding and condensing back into the liquid phase.
Outlines
💧 Understanding Vapor Pressure
This paragraph introduces the concept of vapor pressure using the example of water in a closed beaker. Initially, with no water vapor present, the partial pressure is zero. As the temperature increases, water molecules evaporate, leading to a rise in the partial pressure. The process continues until the rate of evaporation equals the rate of condensation, establishing an equilibrium. At this point, the partial pressure is defined as the vapor pressure, which is a specific value at a given temperature. The paragraph emphasizes the distinction between the partial pressure, which can vary, and the vapor pressure, which is a fixed value at equilibrium.
🌡️ Temperature and Vapor Pressure Relationship
This paragraph explores the relationship between temperature and the vapor pressure of water. It provides specific vapor pressures at different temperatures, illustrating that as temperature increases, so does the vapor pressure. The discussion leads to the definition of the normal boiling point, which is the temperature at which the vapor pressure equals atmospheric pressure (760 torr at sea level). The Clausius-Clapeyron equation is mentioned as a formula that relates vapor pressure and temperature, and it is noted that the full explanation of this formula will be provided in a full version of the video. Additionally, the paragraph discusses how elevation affects atmospheric pressure and, consequently, the boiling point of water, demonstrating that boiling points decrease with increasing elevation.
🏔️ Elevation's Impact on Boiling Point
This paragraph delves into how elevation impacts the boiling point of substances, particularly water. It explains that as elevation increases, atmospheric pressure decreases, leading to a lower boiling point. This is illustrated with examples of boiling points at different elevations, such as at the top of a mountain and in a valley. The paragraph emphasizes that boiling water at higher elevations requires less energy due to the lower boiling point, while in valleys below sea level, more energy is needed to reach the boiling point. The inverse relationship between elevation and boiling point is highlighted, along with the corresponding decrease in atmospheric pressure.
Mindmap
Keywords
💡Vapor Pressure
💡Partial Pressure
💡Evaporation
💡Condensation
💡Equilibrium
💡Temperature
💡Clausius-Clapeyron Equation
💡Normal Boiling Point
💡Atmospheric Pressure
💡Elevation
Highlights
Vapor pressure is the equilibrium pressure at which the rate of evaporation equals the rate of condensation.
The partial pressure of water starts at zero in a closed system and increases as water evaporates.
Evaporation rate is temperature-dependent, increasing with higher temperatures.
At 25 degrees Celsius, the partial pressure of water reaches 15 torr after some time.
Evaporation and condensation occur simultaneously, with the rate of evaporation initially exceeding condensation.
Vapor pressure of water at 23.8 torr is reached when the rates of evaporation and condensation are equal.
Vapor pressure is a specific value at a given temperature, unlike partial pressure which can vary.
The vapor pressure of water has a unique value at each temperature, such as 17.5 torr at 20 degrees Celsius.
At 100 degrees Celsius, the vapor pressure of water equals atmospheric pressure, which is 760 torr.
The normal boiling point of a liquid is the temperature at which its vapor pressure equals atmospheric pressure.
The relationship between temperature and vapor pressure is not linear, with pressure increasing at a greater rate as temperature rises.
The Clausius-Clapeyron equation relates vapor pressure and temperature, useful for calculating new vapor pressures at different temperatures.
At higher elevations, atmospheric pressure decreases, leading to a lower boiling point of water.
At lower elevations or in valleys, atmospheric pressure is higher, resulting in a higher boiling point for water.
Boiling point and elevation are inversely related, with the boiling point decreasing as elevation increases.
Understanding vapor pressure is crucial for various applications, including predicting the boiling point at different elevations.
Transcripts
Browse More Related Video

2.3 Vapor Pressure, IMFs, and Boiling Point
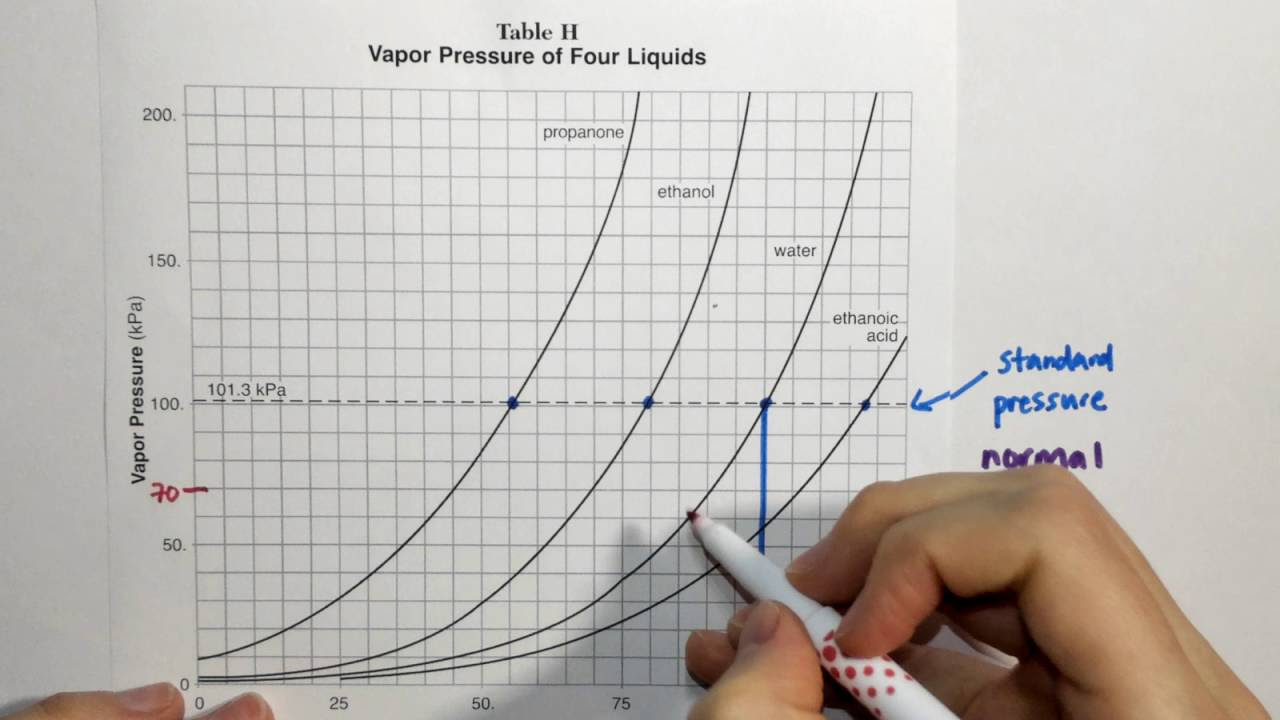
2.4 Reference Table H (Vapor Pressure and Temperature)

Vapor pressure | States of matter and intermolecular forces | Chemistry | Khan Academy

Phase Changes, Heats of Fusion and Vaporization, and Phase Diagrams

Relative Humidity - Dew Point, Vapor & Partial Pressure, Evaporation, Condensation - Physics

The phase diagram of water
5.0 / 5 (0 votes)
Thanks for rating: