The uncertain location of electrons - George Zaidan and Charles Morton
TLDRThis script delves into the atomic structure, explaining that atoms are defined by protons in their nucleus and surrounded by electrons, which exhibit both particle and wave-like behavior. It discusses the probabilistic nature of electron locations, introducing the concept of orbitals as regions of high electron probability. The script also touches on the significance of electron interactions in shaping the properties of all matter, from simple rocks to complex life forms.
Takeaways
- 🌌 Everything is made up of atoms, which are incredibly tiny particles.
- 🔋 An atom's core consists of protons and usually neutrons, with electrons orbiting around it.
- 🔍 The identity of an atom is determined by the number of protons in its nucleus.
- 🚀 Hydrogen, carbon, and gold are examples of elements distinguished by their proton count.
- 🔬 Our understanding of atomic structure comes from experiments and models, which are subject to change with new evidence.
- 🌀 Electrons exhibit both particle-like and wave-like behavior, depending on the experiment.
- 🎲 The exact location of an electron cannot be pinpointed due to the inherent uncertainty in our model of the electron.
- 📊 We can describe the probability of finding an electron in a given space, known as an orbital.
- 🌀 The energy of an orbital affects its shape and density distribution relative to the nucleus.
- 🔄 Electrons' interactions with each other determine chemical properties and reactions.
- 🌐 The nature of all matter, from simple to complex, is fundamentally atomic.
Q & A
What is the fundamental building block of all matter?
-The fundamental building block of all matter is the atom, which is an extremely tiny particle.
What are the basic components of an atom's core?
-An atom's core is made up of at least one proton, which is positively charged, and typically includes neutrons, which are neutral particles.
How does the number of protons in an atom's nucleus determine its identity?
-The identity of an atom is determined by the number of protons in its nucleus; different numbers of protons define different elements, such as hydrogen with one proton and carbon with six.
Why do we rely on models to understand atomic structure when we cannot directly observe subatomic particles?
-We rely on models because direct observation of protons, neutrons, and electrons is not possible. Models are developed and tested against experimental results, and they evolve as new evidence is gathered.
What is the dual nature of electrons as described in the script?
-Electrons exhibit a dual nature, behaving as both particles, like tiny baseballs, and waves, like water waves, depending on the experiment conducted.
Why can't we precisely determine the location of an electron?
-The uncertainty principle inherent in our model of the electron prevents us from pinpointing its exact location, though we can determine the probability of finding it in a given space.
What are orbitals and how do they relate to the probability of finding an electron?
-Orbitals are shapes chemists use to represent the regions around the nucleus where there is a certain probability, such as 95%, of finding an electron.
Why is the probability threshold for finding an electron set at 95% and not 100%?
-The probability threshold is set at 95% because the probability of finding an electron decreases exponentially with distance from the nucleus but never actually reaches zero, leaving a small but non-zero chance of finding an electron far away.
How do the interactions between electrons from different atoms contribute to the nature of everything around us?
-The interactions between electrons, such as the transfer or sharing of electrons, determine the chemical properties and reactions of elements and compounds, which in turn shape the nature of all matter in the universe.
What is the significance of the atomic level in understanding the complexity of life and the human senses?
-The atomic level is significant because it is the foundation upon which the complexity of life is built, and it determines the properties of substances that we perceive through our senses.
Outlines
🌌 Atomic Structure and Electron Behavior
This paragraph delves into the fundamental makeup of atoms, highlighting the roles of protons, neutrons, and electrons. It explains that the atom's identity is determined by the number of protons in its nucleus, and introduces the concept of atomic models developed through experimentation. The paragraph also touches on the dual nature of electrons as both particles and waves, and the Heisenberg Uncertainty Principle, which prevents us from pinpointing an electron's exact location. Instead, we can only describe the probability of finding an electron within a certain 'orbital' shape. The summary also mentions the exponential decrease in the probability of finding an electron far from the nucleus, and the intriguing possibility that electrons may momentarily exist at vast distances from their atoms.
Mindmap
Keywords
💡Atom
💡Core
💡Proton
💡Neutron
💡Electron
💡Orbitals
💡Electron Cloud
💡Chemical Bonding
💡Wave-Particle Duality
💡Quantum Mechanics
💡Atomic Structure
Highlights
Atoms are the fundamental building blocks of all matter, consisting of a nucleus and electrons.
The nucleus contains protons, which determine an atom's identity, and typically neutrons as well.
Electrons, which are negatively charged, orbit the nucleus and are central to chemical reactions.
The atomic model has evolved over time, with experiments continually refining our understanding.
Electrons exhibit wave-particle duality, behaving as both particles and waves.
The Heisenberg uncertainty principle implies we cannot precisely determine an electron's position.
Probability distributions describe the likelihood of finding an electron in a particular region around the nucleus.
Chemists use 'orbitals' to represent regions with a high probability of finding an electron.
Orbital shapes and energy levels influence the behavior and interactions of electrons.
Electrons with higher energy levels are found further from the nucleus.
The probability of finding an electron decreases exponentially with distance from the nucleus but never reaches zero.
Electrons can be found at the other end of the universe for an extremely short time due to quantum mechanics.
Electron interactions with other atoms' electrons are the basis of chemical bonds and reactions.
Chemical bonds involve the transfer or sharing of electrons between atoms.
The atomic level determines the properties of all matter, from simple rocks to complex life forms.
Chemistry is the study of how electrons interact within and between atoms, shaping the nature of all things.
Transcripts
Browse More Related Video
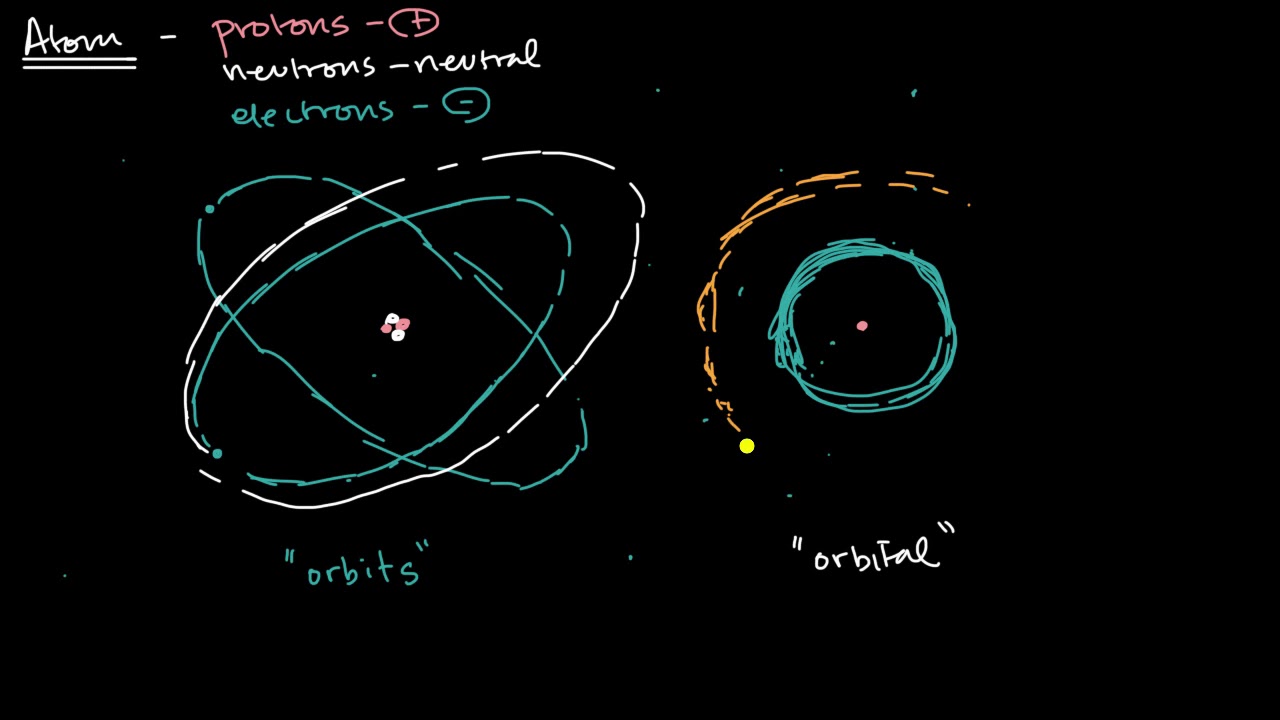
Shells, subshells, and orbitals | Atomic structure and properties | AP Chemistry | Khan Academy

Atomic Structure full topic

Quantum Mechanics and the Schrödinger Equation
Atomic radius trends on periodic table | Periodic table | Chemistry | Khan Academy

AT&T Archives: Matter Waves, Holden and Germer on Wave Nature and the Davisson-Germer Experiment
4. Wave-Particle Duality of Matter; Schrödinger Equation
5.0 / 5 (0 votes)
Thanks for rating: