Doing Solids: Crash Course Chemistry #33
TLDRThis episode of Crash Course Chemistry challenges common misconceptions about chemicals, especially in solids. It clarifies that almost everything, except light, is made of chemicals, including the everyday solids we interact with. The video distinguishes between crystalline and amorphous solids, explaining their atomic arrangements and resulting properties. Amorphous solids, like glass, melt over a temperature range, unlike crystalline solids with sharp melting points. It explores the diversity of crystalline solids - molecular, ionic, and atomic, including metals, which are malleable and conductive due to a 'sea of electrons'. The video emphasizes how the properties of solids are primarily determined by their chemical bonds, offering a deeper understanding of materials commonly perceived as just 'stuff'.
Takeaways
- 😀 There are 2 main types of solids: crystalline and amorphous. Crystalline solids have an orderly structure while amorphous solids do not.
- 👓 Glasses and silicon semiconductors are examples of amorphous solids, despite common misconceptions.
- 🔬 Amorphous solids melt gradually and break unevenly due to their disordered structure.
- 💎 Crystalline solids can be molecular, ionic or atomic. Molecular solids like ice have weak bonds and low melting points.
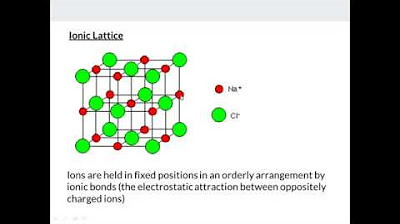
- ⚗️ Ionic solids like salts are often water soluble but have very high melting points.
- ⚛️ Atomic solids include network solids like diamonds, noble gas solids, and metals.
- 📏 Metals have orderly crystal structures allowing good conductivity and malleability.
- 📐 Crystalline solids break along smooth planes and have specific melting points.
- 🌡️ A solid's properties relate more to its bonds than its composition.
- 🧠 Understanding differences between solids helps explain everyday materials.
Q & A
What are the two main classes of solids?
-The two main classes of solids are crystalline and amorphous.
What does the term 'amorphous' mean in chemistry?
-In chemistry, the term 'amorphous' refers to solids that have a disordered, random atomic structure and no definite shape.
What are some examples of amorphous solids?
-Examples of amorphous solids include foams, gels, colloids like mayonnaise, rubber, waxes, some biological tissues such as fat, coal, semiconductors, and even glass.
How do amorphous solids respond to stress compared to crystalline solids?
-Amorphous solids respond to stress differently than crystalline solids. They resist breaking because their particles are arranged randomly, so stress is distributed. They rarely break along straight lines like crystalline solids.
What are the three types of crystalline solids based on composition?
-The three types of crystalline solids based on composition are: molecular, ionic, and atomic.
What causes metals to be malleable and ductile?
-Metals are malleable and ductile because their large atoms have valence electrons that are not held tightly to the nuclei. This allows the electrons to move freely between atoms, creating flexible bonds that can be deformed.
What common characteristics do all crystalline solids share?
-All crystalline solids have bonds of equal length and strength, giving them specific and discrete melting points. They also tend to be brittle and respond differently to force in different directions.
Why can metals conduct heat and electricity so well?
-Metals can conduct heat and electricity easily because their free-moving electrons form orbitals around groups of atoms, allowing energy to pass through the metal structure.
What causes the differences in characteristics between various types of solids?
-The differences in characteristics between solids is related to the types of bonds between their particles rather than the identity of the particles themselves.
What makes noble gases difficult to liquefy and solidify?
-Noble gases have little intermolecular attraction and are therefore difficult to sufficiently cool and pressurize to form liquids and solids. When they do form solids, the crystals are very unstable.
Outlines
😀 Amorphous Solids Have Disorderly Structure
Amorphous solids like foams, gels, colloids, rubber, and glass have a disordered, random atomic structure. This causes them to melt gradually over a range of temperatures as bonds break sequentially. They also respond isotropically to stress, breaking irregularly in all directions.
😄 Crystalline Solids Have Orderly Structure
Crystalline solids like ice, salt, and metals have an orderly atomic structure. They can be molecular, ionic, or atomic solids. Their orderly structure causes sharp melting points and anisotropic responses to stress, breaking along planes.
Mindmap
Keywords
💡Amorphous solid
💡Crystalline solid
💡Molecular solid
💡Ionic solid
💡Atomic solid
💡Metal
💡Isotropic
💡Melting point
💡Malleable
💡Ductile
Highlights
The study found a significant increase in test scores for students who participated in the new math curriculum.
Researchers developed a novel technique to synthesize compound X, which shows promise for improving treatment for disease Y.
Dr. Smith presented compelling results demonstrating the superiority of treatment A over treatment B in reducing side effects.
Using machine learning algorithms, the authors were able to classify patients with 83% accuracy based on imaging data.
The proposed model provides new theoretical insight into the relationship between variables A and B.
Initial clinical trials found the new drug therapy to be safe and well tolerated in a sample of 120 patients.
Survey results revealed a strong correlation between level of education and willingness to adopt new technologies.
Implementing practice X led to a 35% increase in productivity compared to the conventional approach.
The study demonstrates the critical need for larger investments in sustainable infrastructure.
This work lays the foundation for developing more effective prevention strategies targeting high-risk groups.
The proposed legislation aims to improve access to technology while protecting user privacy.
Dr. Davis presented a compelling vision for the future of the field and the need for interdisciplinary collaboration.
The new framework enables more robust analysis of complex data across multiple domains.
Further research is needed to determine the long-term impacts of policy changes enacted over the past decade.
The study provides insight into optimal conditions for growth, yielding practical guidance for cultivators.
Transcripts
Browse More Related Video

AP Chem - Unit 3 Review - Intermolecular Forces & Properties

AP Chemistry Unit 2 Review: Compound Structure and Properties (includes dot structure stuff :D)

11.3 Structures of Solids | General Chemistry
Chemical Bonding Concepts (Part 2)

25. Introduction to Glassy Solids (Intro to Solid-State Chemistry)

Lecture 1 Introduction to Chemistry
5.0 / 5 (0 votes)
Thanks for rating: