AP Chemistry Unit 2 Review: Compound Structure and Properties (includes dot structure stuff :D)
TLDRThe video script is an educational discourse on various chemistry concepts, focusing on molecular and ionic compound structures and properties. It begins with an introduction to chemical bonds, explaining ionic bonds, which form between elements from the first two and last two columns of the periodic table, and their formation through the transfer of electrons, as exemplified by sodium (Na) and chlorine (Cl). Coulomb's law is then introduced to quantify the strength of these ionic bonds, with factors such as charge and distance affecting the bond's energy and lattice energy, which in turn influences a compound's boiling and melting points, as well as solubility. The script moves on to covalent bonds, which are formed between nonmetals and involve the sharing of electrons, and metallic bonds, characterized by a 'sea of electrons' that allow metals to conduct electricity. Electronegativity is also discussed, detailing how it affects bond polarity and type, with examples provided to illustrate the concepts. The script further explores different types of solids, including molecular, network covalent, ionic, and metallic compounds, each with distinct properties and behaviors. Lastly, the principles of Lewis dot structures, formal charges, resonance, and hybridization are explained, offering insights into molecular geometry and the arrangement of electrons in molecules. The summary concludes with a note on valence shell electron pair repulsion (VSEPR) theory, which predicts molecular geometry based on electron pair repulsion. The script is designed to be informative and accessible, aiming to clarify complex chemistry topics for the viewer.
Takeaways
- 🔬 Ionic bonds form between elements in the first two columns and those in the last two columns of the periodic table, resulting in a complete transfer of electrons from one atom to another.
- 📐 Coulomb's law (k * q1 * q2 / r^2) describes the force between charged particles, which is crucial for understanding the strength of ionic bonds and their resulting lattice energy.
- 🔥 Lattice energy is a measure of the strength of ionic bonds and is influenced by charge and distance between ions; higher charges and shorter distances result in stronger ionic bonds.
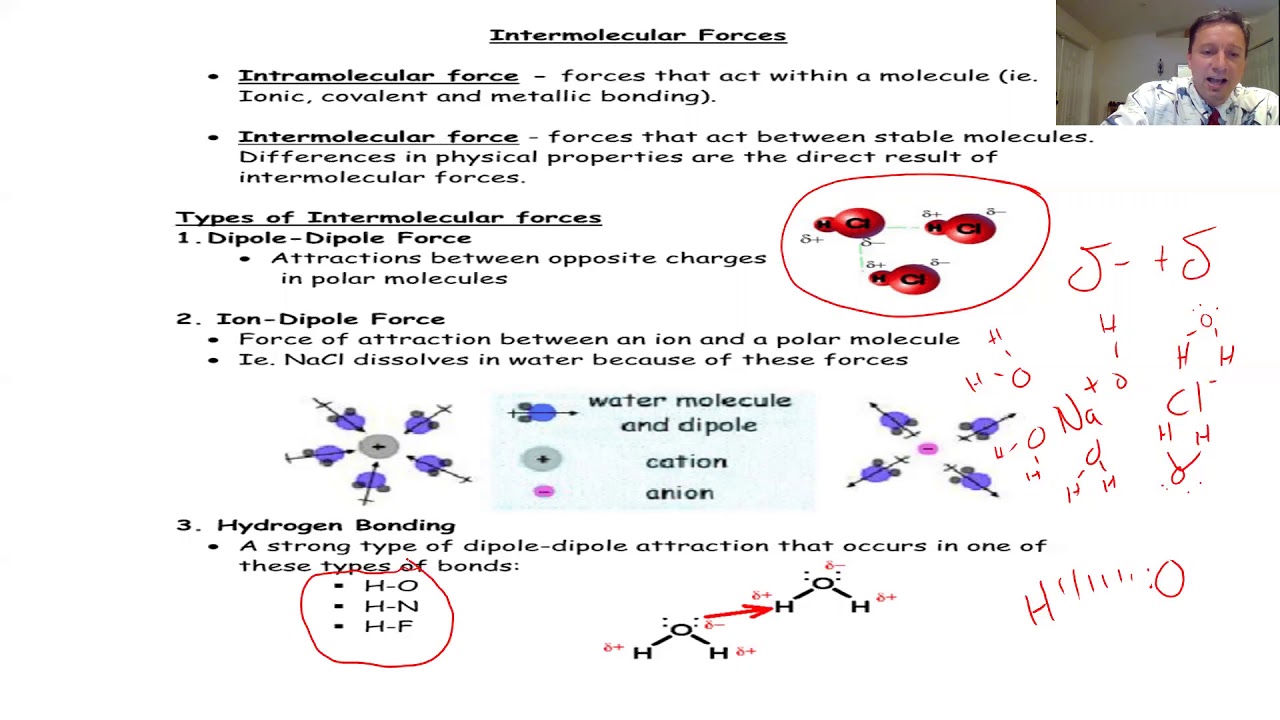
- 🧊 Molecular compounds have low melting and boiling points due to weak intermolecular forces, which are only partial charges as opposed to the full charges in ionic compounds.
- 💧 Covalent bonds are formed between nonmetals and involve the sharing of electrons, creating a stronger bond than ionic bonds because both atoms are holding onto the shared electrons.
- 🌐 Network covalent solids, like diamond, have extremely high melting points and are very hard because they consist of a network of covalently bonded atoms.
- ⚡ Metallic bonds are characterized by a 'sea of electrons' that are free to move around, which gives metals their characteristic properties, such as electrical conductivity and malleability.
- 🤝 Alloys are mixtures of metals that can have different properties from their constituent elements, and they can be interstitial or substitutional, depending on how the atoms are arranged within the alloy structure.
- 📈 Electronegativity differences between atoms determine the type of bond formed: nonpolar covalent bonds (small electronegativity difference), polar covalent bonds (moderate difference), and ionic bonds (large difference).
- 🏗 The type of solid (ionic, molecular, covalent network, or metallic) is determined by the type of chemical bonds present within the structure.
- 📊 Lewis dot structures, formal charges, and resonance structures are important tools for visualizing and understanding the distribution of electrons in molecules.
Q & A
What is the primary focus of Unit 2 in AP Chemistry?
-Unit 2 of AP Chemistry primarily focuses on molecular and ionic compound structure and properties, including topics such as hybridization and polarity.
What are the two general columns in the periodic table that typically form ionic bonds?
-Ionic bonds generally form between elements in the first two columns (usually metals) and elements in the last two columns (usually nonmetals or polyatomic ions).
How does Coulomb's law relate to the strength of ionic bonds?
-Coulomb's law describes the force between two charged particles as directly proportional to the product of their charges and inversely proportional to the square of the distance between them. It is used to explain how the strength of ionic bonds increases with greater charge and decreases with increasing distance between ions.
What property of ionic compounds can be determined by looking at lattice energy?
-The relative boiling points of ionic compounds can be determined by looking at lattice energy, as higher lattice energy indicates stronger bonds and thus higher boiling points.
How do covalent bonds differ from ionic bonds in terms of electron sharing?
-In covalent bonds, two nonmetals share electrons between them, whereas in ionic bonds, one atom completely transfers its electron(s) to another, resulting in a charged ion.
What is a characteristic feature of metallic bonding?
-Metallic bonding is characterized by a 'sea of electrons' where the electrons are delocalized and can move freely throughout the metal structure, leading to properties like electrical conductivity.
How does electronegativity influence the type of bond formed between two atoms?
-Electronegativity determines whether a bond is nonpolar covalent (equal electronegativity), polar covalent (small difference in electronegativity), or ionic (large difference in electronegativity).
What are the differences between molecular solids and network covalent solids?
-Molecular solids have covalent bonds within molecules but weak intermolecular forces between them, leading to low melting and boiling points. Network covalent solids have covalent bonds extending throughout the structure, resulting in very high melting and boiling points and hardness.
Why are ionic compounds soluble in water?
-Ionic compounds are soluble in water because the polar nature of water molecules can surround and separate the ionic compound's ions, allowing them to dissolve.
What is the significance of the term 'resonance' in chemistry?
-Resonance refers to the phenomenon where a single structure cannot fully represent the bonding in a molecule due to the rapid and continuous shifting of electrons, resulting in multiple resonance structures that together describe the true nature of the molecule.
How does valence shell electron pair repulsion (VSEPR) theory help predict molecular geometry?
-VSEPR theory helps predict molecular geometry by considering the repulsion between electron pairs in the valence shell of atoms, which arrange themselves to minimize repulsion and thus determine the shape of the molecule.
What is the concept of hybridization in chemistry?
-Hybridization is the mixing of atomic orbitals in an atom to form new equivalent hybrid orbitals that are suitable for the pairing of electrons to form chemical bonds, allowing for various molecular geometries.
Outlines
🔬 Chemistry Concepts: Ionic Bonds and Lattice Energy
The paragraph introduces the topic of AP Chemistry, specifically focusing on molecular and ionic compound structures and properties. It covers the concept of ionic bonds, which form between elements in the first two columns of the periodic table and those in the last two columns. The paragraph explains how ionic bonds involve the transfer of electrons, creating oppositely charged ions that attract each other. Coulomb's law is introduced to quantify the force between charged particles, and the concept of lattice energy is discussed in relation to the strength of ionic bonds. The summary also touches on how lattice energy affects the boiling points and solubility of ionic compounds.
🤝 Covalent and Metallic Bonds: Understanding Electron Sharing
This section delves into covalent bonding, where electrons are shared between nonmetal atoms, and metallic bonding, characterized by a 'sea of electrons'. Covalent bonds are stronger than ionic bonds because both atoms hold onto the shared electrons. The paragraph explains how electronegativity influences the type of covalent bond formed, whether nonpolar, polar, or ionic. It also discusses the properties of different types of solids, such as molecular compounds with low melting and boiling points, and network covalent solids that are extremely hard due to the interconnected covalent bonds.
💠 Ionic Compounds and Their Physical Properties
The focus shifts to ionic compounds, which are formed from the electrostatic attraction between ions. The paragraph uses NaCl as an example to illustrate how ionic compounds form a lattice structure. It is mentioned that ionic compounds are soluble in water due to their ionic charges and can conduct electricity when dissolved or in a molten state. The brittleness of ionic compounds is also explained, which is a result of the lattice structure being easily disrupted.
🌟 Metallic Compounds: Properties and Alloys
The paragraph discusses metallic compounds, which are characterized by a 'sea of electrons' that contribute to their conductivity and malleability. It is noted that metals are generally not soluble in water and do not conduct electricity in their solid state outside of water. The concept of alloys is introduced, with examples like brass (made from copper and zinc) and bronze (made from copper and tin), highlighting how alloys can have different properties from their constituent metals.
📚 Lewis Dot Structures and Formal Charges
This section covers how to draw Lewis dot structures for molecules like CO2 and O3, explaining the process of placing electrons around atoms to satisfy the octet rule. Formal charges are introduced as a way to predict the most stable electron configuration, with a goal of achieving a formal charge of zero. The concept of resonance is also touched upon, where a bond shifts rapidly between different positions, and bond order is calculated as an average over all resonance structures.
🧬 Valence Shell Electron Pair Repulsion (VSEPR) and Hybridization
The final paragraph explores VSEPR theory, which predicts molecular geometry based on the repulsion between electron pairs in the valence shell. Different geometries such as linear, trigonal planar, tetrahedral, trigonal bipyramidal, and T-shaped are described, along with the concept of lone pairs influencing molecular shape. Hybridization is explained as the process of combining atomic orbitals to form new hybrid orbitals that allow for the formation of different types of bonds, with examples of sp, sp2, sp3, and sp3d hybridization.
Mindmap
Keywords
💡Ionic Bond
💡Coulomb's Law
💡Lattice Energy
💡Covalent Bond
💡Metallic Bond
💡Electronegativity
💡Molecular Solid
💡Network Covalent Solid
💡Ionic Solid
💡Lewis Dot Structure
💡Resonance
💡Hybridization
💡VSEPR Theory
Highlights
Introduction to molecular and ionic compound structure and properties, including hybridization and polarity.
Explanation of ionic bonds, their formation between elements in the first two and last two columns of the periodic table, and exceptions to this rule.
Coulomb's law and its significance in determining the strength of ionic bonds based on charge and distance.
Lattice energy's role in comparing the strength of ionic bonds and its correlation with melting and boiling points.
Solubility of ionic compounds in water and their ability to conduct electricity when dissolved.
Covalent bonds, their formation between nonmetals, and the concept of electron sharing.
Differences between intramolecular forces within a molecule and intermolecular forces between different molecules.
Characteristics of metallic bonds, including the 'sea of electrons' model and why metals are good conductors of electricity.
Electronegativity and its impact on bond polarity, with examples of nonpolar and polar covalent bonds.
The relationship between electronegativity differences and the formation of ionic bonds.
Types of solids related to chemical bonding, including molecular, network covalent, ionic, and metallic solids.
Properties of molecular compounds, such as low boiling and melting points, and their ease of sublimation.
Network covalent solids, their exceptional hardness, and examples like diamond and silicon dioxide.
Explanation of why ionic compounds are brittle and the significance of their lattice structure.
Properties of metallic compounds, including malleability, electrical conductivity, and the concept of alloys.
Lewis dot structures for representing covalent bonds and the importance of valence electrons in bonding.
Formal charges in Lewis structures and strategies for minimizing them by placing negative charges on more electronegative elements.
Resonance structures and how they represent the dynamic nature of certain molecules, like ozone.
VSEPR theory and its use in predicting molecular geometry based on the repulsion of electron pairs.
Hybridization of atomic orbitals and its role in forming different types of bonds, such as SP, SP2, SP3, and SP3D2.
Transcripts
Browse More Related Video
AP Chemistry Unit 2 Review

AP Chem - Unit 2 Review - Molecular & Ionic Compound Structure and Properties

Introduction to Ionic Bonding and Covalent Bonding

Ionic, covalent, and metallic bonds | Chemical bonds | Chemistry | Khan Academy

General Chemistry Review for Organic Chemistry

Types of Chemical Bonds - AP Chem Unit 2, Topic 1
5.0 / 5 (0 votes)
Thanks for rating: