AP Chem - Unit 3 Review - Intermolecular Forces & Properties
TLDRJeremy Krug's review of AP Chemistry Unit 3 dives into the intricacies of intermolecular forces and their impact on molecular properties. He explains that London dispersion forces, though typically weak, can become significant in larger nonpolar molecules. Dipole-dipole forces are stronger and occur between polar molecules, while hydrogen bonding is a particularly strong force found in molecules with hydrogen atoms bonded to oxygen, fluorine, or nitrogen. These forces correlate with higher boiling and melting points in compounds. Solids vary in properties; ionic solids have high melting points and conduct electricity when dissolved, covalent network solids like diamond are extremely strong, molecular solids have low melting points due to weak intermolecular forces, and metallic solids are malleable and good conductors. The video also covers the Ideal Gas Law, gas mixtures, and the behavior of gases. It touches on the concepts of heterogeneous and homogeneous mixtures, molarity, and solution diagrams. Separation techniques like distillation and chromatography are discussed, along with solubility rules and the effects of the electromagnetic spectrum on molecules. The dual nature of light, the calculation of photon energy, and the application of the Beer-Lambert Law in spectrophotometry round out the comprehensive overview of Unit 3.
Takeaways
- 🔬 All molecules exhibit London dispersion forces, which are usually the weakest but can be stronger in large nonpolar molecules.
- 📌 Polar molecules experience dipole-dipole forces, which are generally stronger than dispersion forces.
- 🔗 Hydrogen bonding is a particularly strong intermolecular force found in molecules with hydrogen atoms bonded to oxygen, fluorine, or nitrogen.
- 🌡️ The strength of intermolecular forces correlates with the compound's boiling and melting points, with hydrogen bonds being the strongest.
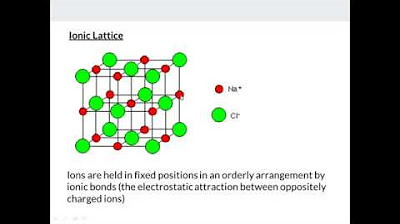
- ⚜️ Ionic solids have high melting points due to strong ionic attractions and are brittle, conductive when dissolved in water.
- 💎 Covalent network solids like diamond are extremely strong due to extensive covalent bonding between atoms.
- 🍬 Molecular solids, such as sugar, have weak intermolecular forces and low melting points because they consist of individual molecules.
- 🔩 Metallic solids are malleable, ductile, and good conductors of electricity due to the presence of a 'sea' of electrons around a metallic core.
- 📏 The Ideal Gas Law, PV=nRT, is fundamental for understanding the behavior of gases, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature.
- 🔄 In a mixture of gases, the total pressure is the sum of the partial pressures of the individual gases, which can be determined by multiplying the mole fraction by the total pressure.
- 📊 Temperature is a measure of the average kinetic energy of molecules, and the Boltzmann distribution helps visualize the range of molecular motion at different temperatures.
- 🧪 Solutions can be homogeneous (uniform distribution) or heterogeneous (visible components), and molarity is a key concept for expressing solution concentration.
Q & A
What type of intermolecular forces do all molecules exhibit?
-All molecules exhibit London dispersion forces, which are usually the weakest intermolecular forces but can be stronger in large molecules.
How do the number of electrons in a molecule affect its intermolecular forces?
-The more electrons a molecule has, the more polarizable it is, leading to stronger London dispersion forces.
What is the relationship between the strength of intermolecular forces and the boiling and melting points of a compound?
-The stronger the intermolecular forces, the higher the boiling and melting points of the compound. London dispersion forces are the weakest, followed by dipole-dipole forces, with hydrogen bonds being the strongest.
Why do ionic solids have high melting points?
-Ionic solids have high melting points due to the strong attractions between oppositely-charged ions, making them brittle and good conductors of electricity when dissolved in water.
How are covalent network solids, such as diamond and silicon dioxide, different from other types of solids?
-Covalent network solids have extremely strong covalent forces, with each atom bonded to multiple other atoms in multiple directions, making them very strong.
What is the primary difference between molecular solids and ionic or covalent network solids?
-Molecular solids consist of individual molecules as units, with relatively weak forces between molecules and relatively low melting points, unlike ionic or covalent network solids.
What property of metals allows them to be malleable, ductile, and good conductors of electricity?
-Metals are malleable and ductile, and they conduct electricity well due to a metallic core surrounded by a sea of electrons.
What is the Ideal Gas Law and how is it expressed mathematically?
-The Ideal Gas Law is expressed as PV=nRT, where P is pressure in atmospheres, V is volume in liters, n is the number of moles of gas, R is the Universal Gas Constant, and T is the temperature in Kelvins.
What is the significance of the Boltzmann distribution in relation to molecular motion?
-The Boltzmann distribution helps visualize the range of motion in molecules at different temperatures, showing that at higher temperatures, a greater fraction of particles are moving faster.
What are the two main types of mixtures, and how do they differ?
-The two main types of mixtures are heterogeneous mixtures, where different components can be seen with the eyes, and homogeneous mixtures, also known as solutions, where all components are uniformly distributed.
How does the rule of thumb 'Like dissolves like' apply to the solubility of compounds?
-'Like dissolves like' means that polar molecules tend to dissolve in polar solvents, often through hydrogen bonding or dipole-dipole forces, while nonpolar molecules tend to dissolve in nonpolar solvents.
What is the Beer-Lambert Law, and how is it used in spectrophotometry?
-The Beer-Lambert Law is often written as A = epsilon b c, where A is absorbance, epsilon is the molar absorptivity, b is the path length, and c is the concentration of the sample. It is used to build a calibration curve to determine unknown concentrations in spectrophotometry.
Outlines
🔬 Intermolecular Forces and Properties in AP Chemistry
Jeremy Krug introduces Unit 3 of AP Chemistry, focusing on intermolecular forces and their impact on a compound's physical properties. He explains that all molecules exhibit London dispersion forces, which are typically weak but can be stronger in large molecules. For nonpolar molecules, these are the only intermolecular forces present. Polar molecules experience dipole-dipole forces, which are generally stronger than dispersion forces. Hydrogen bonding, a very strong intermolecular force, occurs in molecules with hydrogen atoms bonded to electronegative atoms like oxygen, fluorine, or nitrogen. The strength of these forces correlates with the compound's boiling and melting points. The video also covers different types of solids, their properties, and the structure of true solids versus amorphous solids. Krug explains the Ideal Gas Law and its application, the concept of partial pressures in gas mixtures, and graphical relationships between gas properties. Temperature is discussed in relation to the average kinetic energy of molecules, and the behavior of ideal gases is contrasted with real-world gases. The video concludes with a brief mention of the next unit.
🧪 Solutions, Distillation, and Chromatography in AP Chemistry
This paragraph delves into the types of mixtures, with a focus on heterogeneous and homogeneous mixtures, the latter often referred to as solutions. Molarity is introduced as a key concept for discussing solution concentration, defined as the moles of solute per liters of solution. Diagrams are used to illustrate mole ratios and reactions in solutions. The video then covers two primary methods for separating components in solutions: distillation, which separates substances based on different boiling points, and chromatography, which separates components based on their interaction with a column. The 'Like dissolves like' rule is introduced for predicting solubility, and the effects of different parts of the electromagnetic spectrum on molecules are discussed. The dual nature of light as both a wave and a particle (photon) is explained, along with how to calculate the energy of photons. The Beer-Lambert Law is introduced in the context of spectrophotometry, and the importance of building a calibration curve for determining unknown concentrations is emphasized. The video concludes with advice on handling outliers in calibration curves.
📈 Calibration Curves and Unit 3 Conclusion
Jeremy Krug wraps up the review of Unit 3 with a discussion on calibration curves in spectrophotometry. He explains how to plot known concentrations against absorbance to build a calibration curve, which can then be used to determine the concentration of unknown samples. He also addresses common causes for outliers in calibration curves, such as contamination or dilution errors. Krug then invites viewers to join him for the next review session covering Unit 4 of AP Chemistry.
Mindmap
Keywords
💡London Dispersion Forces
💡Dipole-Dipole Forces
💡Hydrogen Bonding
💡Boiling Point and Melting Point
💡Ionic Solids
💡Covalent Network Solids
💡Molecular Solids
💡Metallic Solids
💡Ideal Gas Law
💡Partial Pressure
💡Boltzmann Distribution
💡Heterogeneous and Homogeneous Mixtures
💡Distillation and Chromatography
💡Solubility
💡Electromagnetic Spectrum
💡Spectrophotometry and Beer-Lambert Law
Highlights
All molecules exhibit London dispersion forces, which are typically the weakest intermolecular forces but can be stronger in large molecules.
London dispersion forces are the only intermolecular force present in nonpolar molecules and increase in strength with the number of electrons in a molecule.
Polar molecules experience stronger dipole-dipole forces, where the positive pole of one molecule attracts the negative pole of its neighbor.
Hydrogen bonding is a particularly strong intermolecular force observed in molecules with a hydrogen atom bonded to oxygen, fluorine, or nitrogen.
The strength of intermolecular forces correlates with the compound's boiling and melting points, with hydrogen bonds being the strongest.
Ionic solids have high melting points due to strong attractions between oppositely-charged ions and are brittle, conducting electricity when dissolved in water.
Covalent network solids like diamond and silicon dioxide are extremely strong due to each atom being bonded to multiple other atoms in multiple directions.
Molecular solids, exemplified by sugar, have relatively weak forces between molecules and low melting points compared to other types of solids.
Metallic solids are malleable, ductile, and good conductors of electricity due to a metallic core surrounded by a sea of electrons.
True solids are crystalline, while amorphous solids like plastics have a non-crystalline structure with particles very close to each other.
The Ideal Gas Law, PV=nRT, is used to calculate relationships between pressure, volume, the number of moles, and temperature for gases.
The partial pressures of individual gases in a mixture add up to the total pressure, and the partial pressure of a gas is determined by its mole fraction in the container.
Graphs illustrating the relationships between pressure, volume, temperature, and the number of moles of gas are essential for understanding gas behavior.
Temperature is a measure of the average kinetic energy of molecules; higher temperatures result in a greater fraction of particles moving faster.
The Ideal Gas Law is an approximation that works best for gases with little interparticle attraction and small molecular size, like helium, at high temperatures and low pressures.
Mixtures can be heterogeneous, where components are visible, or homogeneous, known as solutions, and molarity is used to express solution concentration.
Distillation and chromatography are primary methods for separating components in solutions based on different boiling points and varying adherence to a column.
The rule of thumb 'Like dissolves like' helps predict solubility, where polar molecules dissolve in polar solvents and nonpolar in nonpolar solvents.
Different parts of the electromagnetic spectrum affect molecules in various ways, such as causing electron transitions or inducing vibrations and rotations.
Light has a dual nature as both a wave and a particle (photon), and its energy can be calculated using the equations c = lambda nu and E = h nu.
The Beer-Lambert Law is used in spectrophotometry to determine unknown concentrations by building a calibration curve based on absorbance and concentration.
Outliers in a calibration curve may indicate contamination or dilution, affecting the accuracy of concentration measurements.
Transcripts
Browse More Related Video

AP Chemistry Unit 2 Review

10.1 Intermolecular Forces | High School Chemistry
AP Chemistry Unit 3 Review: Intermolecular Forces and Properties
Chemical Bonding Concepts (Part 2)

Intermolecular Forces - Hydrogen Bonding, Dipole Dipole Interactions - Boiling Point & Solubility

11.1 Intermolecular Forces | General Chemistry
5.0 / 5 (0 votes)
Thanks for rating: