11.3 Structures of Solids | General Chemistry
TLDRThis educational video script delves into the structures of solids, focusing on cubic unit cells, including simple cubic, body-centered cubic, and face-centered cubic arrangements. It explains atom distribution, coordination numbers, and packing efficiencies. The lesson continues with close packing of spheres, types of solids (molecular, network covalent, ionic, and metallic), and their melting points related to bonding strength. Aimed at simplifying science learning, it's part of a general chemistry series with supplementary resources available.
Takeaways
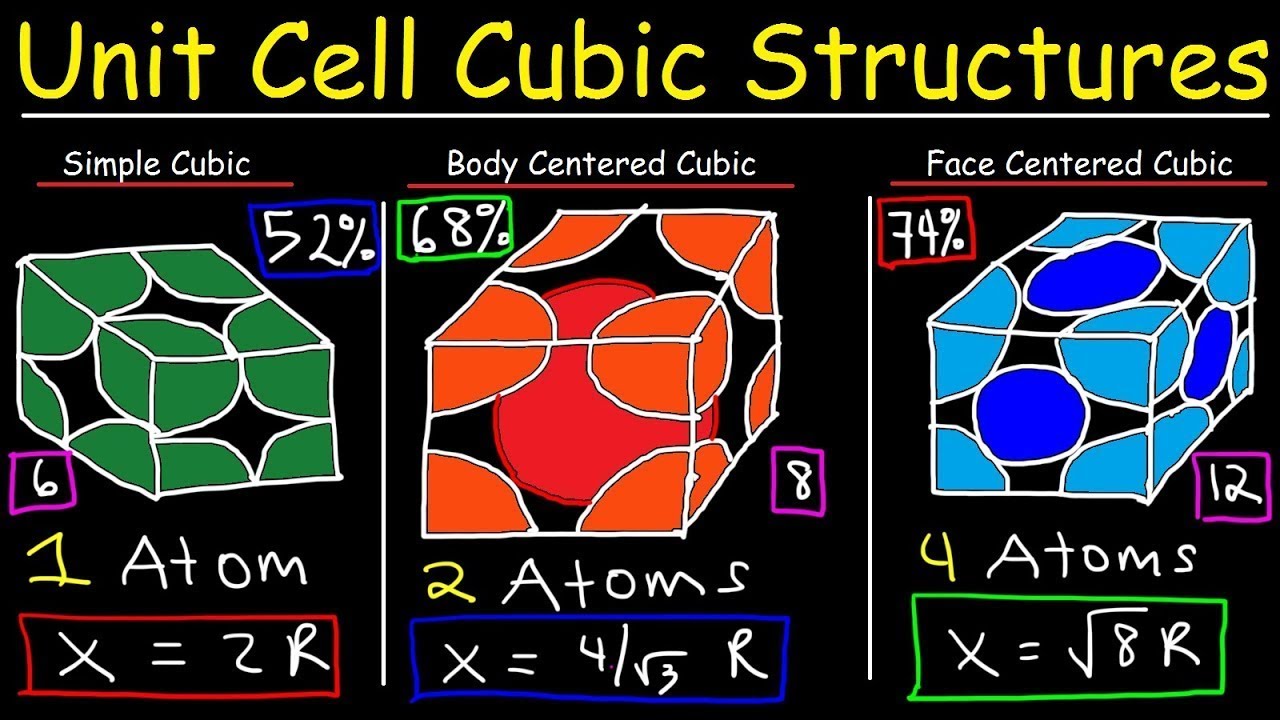
- 📐 The lesson covers three types of cubic unit cells: simple cubic, body-centered cubic, and face-centered cubic, each with different atom positions and counts per unit cell.
- 👀 In simple cubic cells, atoms are only at the corners, while body-centered cubic cells have an additional atom at the center. Face-centered cubic cells have atoms at the center of each face in addition to the corners.
- 🔢 The number of atoms per unit cell is one for simple cubic, two for body-centered cubic, and four for face-centered cubic.
- 🤝 The coordination number, which is the number of nearest neighbors an atom has, varies: 6 for simple cubic, 8 for body-centered cubic, and 12 for face-centered cubic.
- 📏 The edge length of a cubic unit cell (a) is related to the radius of the atoms it contains, with specific relationships for each type of cubic cell.
- 🔍 The packing efficiency of atoms within the unit cells increases from simple cubic (52%) to body-centered cubic (68%) to face-centered cubic (74%).
- 🔄 Close packing of spheres is discussed with hexagonal close-packed and cubic close-packed structures, both of which are common in metals.
- 🔗 The lesson explains that the strength of the bonds in solids affects their melting points, with molecular solids typically having the lowest melting points due to weaker intermolecular forces.
- 💎 Network covalent solids, like diamond and quartz, have very high melting points due to strong covalent bonds within their crystal lattices.
- ⚜️ Ionic solids' melting points are influenced by lattice energy, with larger charges and smaller ions leading to stronger ionic bonds and higher melting points.
- 🌟 Metallic solids show varying melting points, with trends not as straightforward as other types of solids, especially considering the transition metals' unique behavior.
Q & A
What are the three types of cubic unit cells discussed in the script?
-The three types of cubic unit cells are simple cubic, body-centered cubic, and face-centered cubic.
How many atoms are in a simple cubic unit cell?
-There is one atom per simple cubic unit cell, which is shared among the eight corners of the cube.
What is the coordination number in a simple cubic unit cell?
-The coordination number in a simple cubic unit cell is 6, as each atom is surrounded by six nearest neighbors in the same layer.
How is the coordination number different in a body-centered cubic unit cell compared to a simple cubic unit cell?
-In a body-centered cubic unit cell, the coordination number is 8, which is higher than the 6 found in a simple cubic unit cell, due to the presence of an additional atom at the center of the cube.
What is the packing efficiency of a simple cubic unit cell?
-The packing efficiency of a simple cubic unit cell is 52%, indicating the percentage of the volume of the cube that is occupied by atoms.
What is the relationship between the edge length 'a' and the radius 'r' of an atom in a simple cubic unit cell?
-In a simple cubic unit cell, the edge length 'a' is equal to 2 times the radius 'r' of the atom (a = 2r).
What are the two types of close packing of spheres mentioned in the script?
-The two types of close packing of spheres are hexagonal close packing (hcp) and cubic close packing (ccp).
How do the repeating layers differ between hexagonal close packed and cubic close packed structures?
-In hexagonal close packing, there is an alternating set of two layers (ABAB...), while in cubic close packing, there is an alternating set of three layers (ABCABC...).
What are the four types of solids discussed in the script, and how do their melting points generally compare?
-The four types of solids are molecular solids, network covalent solids, ionic solids, and metallic solids. Generally, network covalent solids have the highest melting points, followed by ionic solids, metallic solids have a broad spectrum, and molecular solids usually have the lowest melting points.
How does the melting point of ionic solids relate to their lattice energy?
-The melting point of ionic solids is directly related to their lattice energy; higher lattice energy indicates stronger ionic bonds and thus a higher melting point.
What is a common characteristic of metallic solids that have high melting points?
-Metallic solids with high melting points often have more unpaired electrons available for bonding, which contributes to stronger metallic bonds and higher melting points.
Outlines
📚 Introduction to Solid Structures and Unit Cells
This paragraph introduces the topic of solid structures, focusing on cubic unit cells. It discusses three types: simple cubic, body-centered cubic, and face-centered cubic. The explanation includes where atoms are located within these unit cells, the number of atoms per unit cell, coordination numbers, and packing efficiencies. The paragraph also covers different types of solids, such as molecular, network covalent, ionic, and metallic solids, and touches on the relationship between bonding strength and melting points. The instructor, Chad, welcomes viewers to his science prep channel and mentions that some general chemistry classes may omit this material, so its relevance may vary.
🔍 Detailed Explanation of Cubic Unit Cells
This paragraph delves deeper into the specifics of cubic unit cells, explaining the arrangement of atoms in simple cubic, body-centered cubic, and face-centered cubic structures. It quantifies the number of atoms contributed by the corners and centers of the cells, resulting in 1, 2, and 4 atoms per unit cell respectively. The concept of coordination numbers, which is the count of the nearest neighbors to any given atom, is introduced with examples for each type of cubic cell. The paragraph also establishes the relationship between the edge length of the unit cells and the radius of the atoms they contain.
📐 Geometry of Atomic Packing in Cubic Unit Cells
The focus shifts to the geometric aspects of atomic packing within cubic unit cells. The paragraph explains how the edge length of a cube is related to the radius of the atoms it contains, particularly in face-centered cubic structures. It uses the Pythagorean theorem to derive the relationship between the diagonal of a face and the edge length, leading to the formula for atomic radius in terms of the edge length. The paragraph also addresses the packing efficiency of different cubic unit cells, noting the increase in efficiency from simple cubic to body-centered cubic to face-centered cubic.
🌐 Close Packing of Spheres and Layering in Structures
This paragraph explores the close packing of spheres, specifically hexagonal close packing (hcp) and cubic close packing (ccp). It describes the process of stacking layers of spheres and how the layers alternate to create different structures. The explanation highlights the two repeating layers in hcp (A-B pattern) and the three repeating layers in ccp (A-B-C pattern). The paragraph emphasizes the high packing efficiency of these structures, which are common in metals, and the importance of understanding the layering process for grasping the concept of close packing.
🔬 Bonding in Solids and the Impact on Melting Points
The paragraph discusses the four types of solids—molecular, network covalent, ionic, and metallic—and how the strength of the bonds or interactions within these solids affects their melting points. It explains that molecular solids, held together by weaker intermolecular forces, generally have lower melting points compared to network covalent solids, which are held together by stronger covalent bonds within a crystal lattice. The paragraph also introduces the concept of lattice energy in ionic solids and how it correlates with higher melting points due to stronger ionic bonds.
🔬 Trends in Melting Points of Ionic and Metallic Solids
This paragraph continues the discussion on the melting points of solids, focusing on ionic and metallic solids. It explains that for ionic solids, the size and charge of the ions influence the strength of the ionic bonds and the lattice energy, which in turn affects the melting point. Smaller ions with higher charges result in stronger bonds and higher melting points. The paragraph also addresses the melting points of metallic solids, noting that smaller metals have higher melting points due to shorter and stronger metallic bonds. It also touches on the unique trends observed in transition metals, where melting points increase towards the center of the transition series due to the availability of unpaired electrons for bonding.
📘 Conclusion and Resources for General Chemistry
The final paragraph concludes the lesson by summarizing the key points regarding the types of solids and their melting points. It emphasizes the general trend that molecular solids have the lowest melting points, followed by metallic solids, with network covalent solids and ionic solids having the highest. The paragraph also provides information about additional resources available for general chemistry students, including practice problems, final exam rapid reviews, and a comprehensive master course with over 1200 practice questions. The instructor encourages students to use these resources to prepare for their exams and offers a link for free trials in the description.
Mindmap
Keywords
💡Cubic Unit Cells
💡Coordination Number
💡Packing Efficiency
💡Close Packing of Spheres
💡Molecular Solids
💡Network Covalent Solids
💡Ionic Solids
💡Metallic Solids
💡Melting Point
💡Amorphous Solids
Highlights
Introduction to the structures of solids, focusing on cubic unit cells including simple cubic, body-centered cubic, and face-centered cubic.
Explanation of atomic locations within unit cells and the number of atoms per unit cell.
Discussion on coordination numbers in different cubic unit cells and how they relate to atomic arrangements.
Packing efficiencies of different cubic unit cells and the progression from simple cubic to face-centered cubic.
Introduction to close packing of spheres, including hexagonal close packed and cubic close packed structures.
Differences between hexagonal close packed and cubic close packed structures in terms of repeating layers.
Exploration of bonding in solids, covering molecular, network covalent, ionic, and metallic solids.
Correlation between the strength of bonding and the melting points of different types of solids.
Characteristics of molecular solids and their generally lower melting points due to weaker intermolecular forces.
Network covalent solids, such as diamond and quartz, and their very high melting points due to strong covalent bonds.
Analysis of ionic solids, lattice energy, and the impact of ionic bond strength on melting points.
Metallic solids and the trends in melting points related to the number of valence electrons available for bonding.
Transition metals' unique trends in melting points and the role of unpaired electrons in metallic bonding.
The broad spectrum of melting points in metallic solids, from those that melt easily to those with very high melting points.
Summary of the key characteristics to understand regarding different types of solids and their melting points.
Invitation for feedback and the offer of supplementary materials for further study and practice.
Transcripts
Browse More Related Video
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu
Chemical Bonding Concepts (Part 2)

AP Chem - Unit 3 Review - Intermolecular Forces & Properties

AP Chemistry Unit 2 Review: Compound Structure and Properties (includes dot structure stuff :D)
2022 Live Review 7 | AP Chemistry | Bonding and the Condensed State

[H2 Chemistry] 2023 Topic 2 Chemical Bonding 1
5.0 / 5 (0 votes)
Thanks for rating: