The Periodic Table: Crash Course Chemistry #4
TLDRThe video tells the story of Dmitri Mendeleev, a Russian chemist who overcame hardship to revolutionize chemistry by creating the periodic table. After his father's death, Mendeleev's mother rode 1200 miles on horseback so he could attend university. Though rejected at first, his persistence earned him acceptance. His studies allowed him unique insights on element properties and relationships. Obsessed with finding order, he left gaps for undiscovered elements, accurately predicting their attributes. This theoretical framework highlighted cosmic patterns and brought new understanding. Though others had similar ideas, Mendeleev stood out through his obsessive, almost religious fervor. The resulting periodic table became a guiding light for future discoveries.
Takeaways
- 😢 Dmitri Mendeleev overcame a tragic childhood to become a pioneering Russian chemist
- 👨🔬 Mendeleev organized the known elements into the first periodic table
- 🔢 The periodic table revealed patterns and properties of elements
- 💡 Mendeleev predicted the existence and properties of undiscovered elements
- 🔭 The periodic table provided a guide to future discoveries in chemistry
- ⚛️ The 'why' behind periodicity was later explained by the discovery of electrons
- 📏 Other chemists envisioned alternative layouts for the periodic table
- 😠 Mendeleev disliked ideas he couldn't see directly with his own eyes
- 📜 Our modern periodic table has some flaws in its layout
- 🙏 Mendeleev perhaps saw divine pattern in his periodic table
Q & A
Who was Dmitri Ivanovich Mendeleev?
-A Russian chemist who created the periodic table of elements by organizing elements according to their atomic weights and chemical properties.
What motivated Mendeleev to create the periodic table?
-He became obsessed with finding order and patterns among the known elements at the time. He shuffled element cards on his desk for days until he realized he was missing some yet-to-be-discovered elements.
What did Mendeleev predict about the missing elements in his table?
-He predicted their properties based on the patterns he observed, leaving gaps in his table for them. When some were discovered later, his predictions were proven remarkably accurate.
How did Mendeleev stand out from other chemists working on organizing elements?
-He knew the elemental data comprehensively and spent more time working on finding relationships between elements. He also realized the periodic table had profound importance beyond just categorizing elements.
What groups of elements did Mendeleev identify in his table?
-Alkali metals, alkaline earth metals, transition metals, metalloids/nonmetals, halogens and noble gases. The categories reflected their shared properties and reactivities.
What didn't Mendeleev know about when he created his table?
-He didn't know about electrons, so he couldn't explain why elements were periodic. He actually denied the existence of atoms.
How did others visualize the periodic table differently than Mendeleev?
-De Chancourtois pictured it as a cylinder wrapping elements periodically. Others proposed circular tables so similar elements would be adjacent.
What improvements were later made to Mendeleev's table design?
-Rare earth metals were integrated instead of separated out. Tables were circularized to eliminate artificial separations between related elements.
Why was Mendeleev's table an important scientific guide?
-It organized existing knowledge and provided a framework to predict undiscovered elements and their properties decades before they were found.
What provided the answer to why elements were periodic in properties?
-The discovery of electrons and their arrangement in shells around atom nuclei explained the periodic repetitions in properties.
Outlines
😯 The amazing background of how Mendeleev created the periodic table
Mendeleev had an incredible life story, from growing up poor in Siberia to traveling across Russia with his mother to eventually attend university. He studied chemistry extensively and realized there were patterns in the properties of elements based on atomic weight. He became obsessed with organizing them and predicting the properties of undiscovered elements, leaving gaps in his early periodic tables. He was so confident that he corrected the properties of newly discovered elements when they didn't match his predictions.
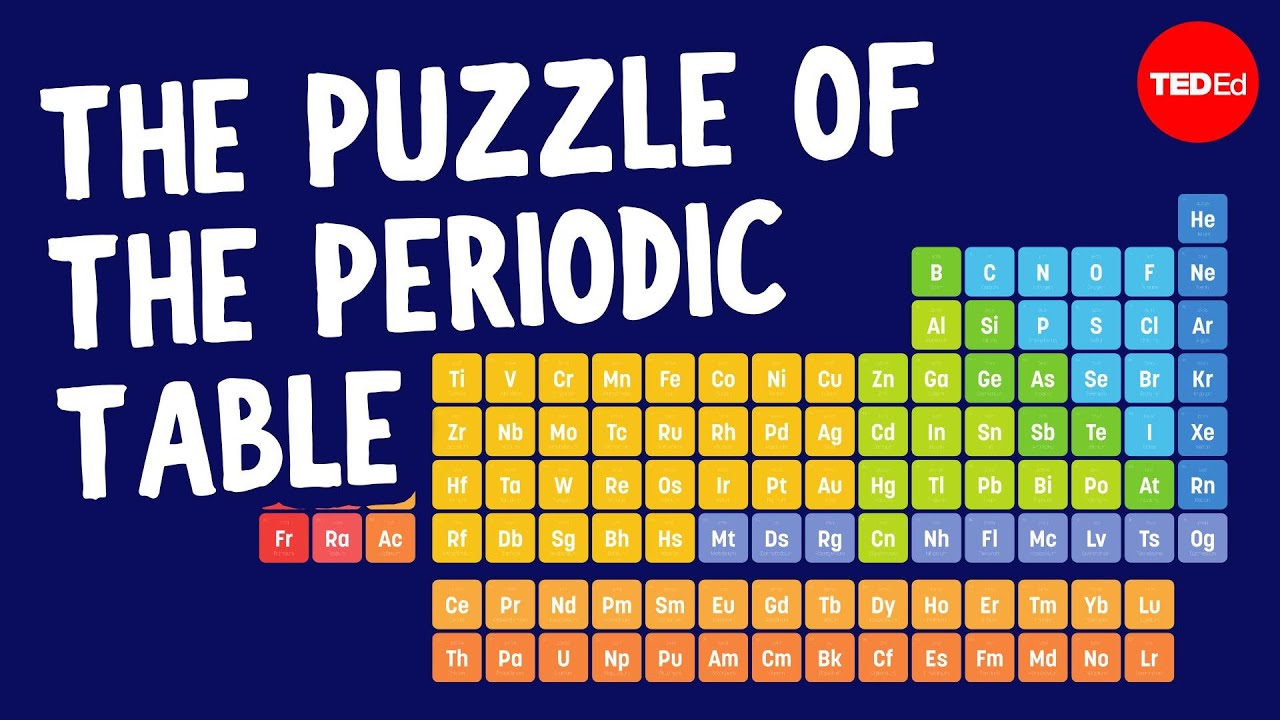
👨🔬 How the modern periodic table is organized into groups
The periodic table arranges elements into groups with similar properties. The left side contains very reactive metals that form positive ions. The middle section has transition metals like iron and gold. On the right are the reactive nonmetals called halogens that form negative ions. At the bottom are lanthanides and actinides. And on the far right, the inert noble gases.
📈 Other forms and improvements for the periodic table
There are other ways to visualize the periodic table, like a 3D cylinder or spiral wrapping around. The table keeps improving over time to better reflect the relationships between elements. The current common table separates out the lanthanides and actinides when they should be integrated. Making it circular with fluorine connected to sodium across the circle would be more accurate.
Mindmap
Keywords
💡periodic table
💡element
💡atomic weight
💡alkali metal
💡halogen
💡transition metal
💡metalloid
💡lanthanide
💡actinide
💡noble gas
Highlights
Mendeleev became obsessed with organizing the elements and their properties into a table
He realized relationships between elements had to do with atomic weights being periodic, not just increasing
Mendeleev left gaps in his early periodic tables, predicting the existence and properties of undiscovered elements
When new elements were discovered, Mendeleev would argue if data didn't match his predictions about them
Mendeleev identified groups of elements that react similarly, like alkali metals and halogens
Metals make up the majority of elements; they are malleable, conductive, and important but unreactive
Mendeleev was obsessed, knew the data intensely well, and saw cosmic importance in periodicity
He published his table simply as part of a textbook he wrote to generate needed cash
At least 6 people published on periodicity around the same time, but Mendeleev stood out
Another contemporary visualized a 3D cylindrical periodic table, but couldn't publish the concept
Answering why periodicity occurs requires understanding electrons, which Mendeleev likely would've hated
Our modern table separates out lanthanides/actinides; a circular form would be more accurate
After Mendeleev, the scientific community intensely questioned why periodicity occurs
The electron ended up answering that question, though Mendeleev denied atoms existed
Mendeleev created a guide to help future chemists understand undiscovered elements
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: