AP Chemistry Unit 3 Review: Intermolecular Forces and Properties
TLDRThe video script offers an engaging walkthrough of Unit 3 in 'Baby Chemistry,' focusing on intermolecular forces and their impact on molecular properties. The presenter, Cara, explains the hierarchy of these forces from the weakest (London dispersion forces) to the strongest (hydrogen bonds), highlighting their role in phase changes and surface tension. The script delves into the importance of understanding intermolecular forces for grasping concepts like phase transitions, melting, and boiling points. It also touches on the ideal gas law, kinetic molecular theory, and the photoelectric effect, providing a comprehensive overview of fundamental chemistry concepts.
Takeaways
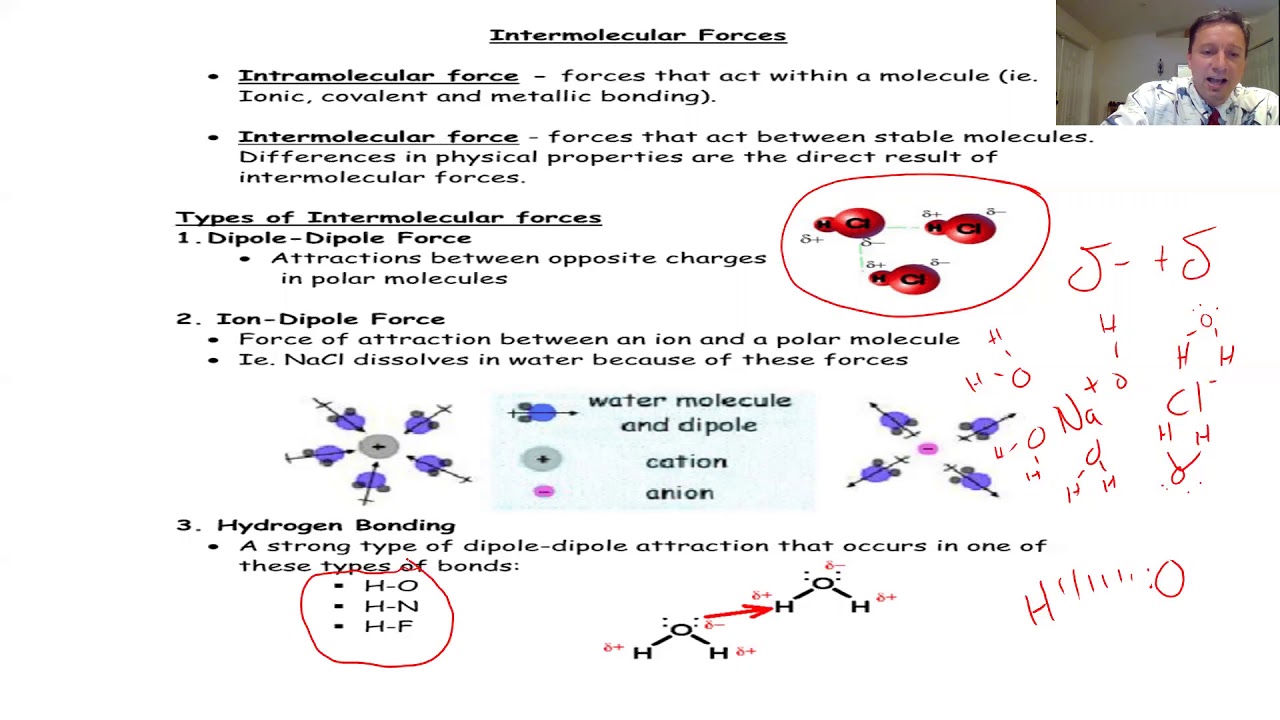
- 🔬 The video discusses intermolecular forces, which are the forces between different molecules and are categorized from weakest to strongest as London dispersion forces, dipole-dipole forces, and hydrogen bonds.
- 🔬 London dispersion forces are present in all molecules and are caused by temporary fluctuations in electron distribution, making them weak forces.
- 🔬 Dipole-dipole forces occur in polar molecules and are stronger than London dispersion forces because they are permanent and involve a consistent attraction between polar molecules.
- 🔬 Hydrogen bonds are a special type of dipole-dipole interaction that occurs when hydrogen is bonded to highly electronegative elements like nitrogen, oxygen, or fluorine, resulting in particularly strong intermolecular forces.
- 🌡️ Phase changes are influenced by intermolecular forces, as energy is required to overcome these forces to transition between solid, liquid, and gas states.
- 🌡️ The strength of intermolecular forces also affects properties such as surface tension and capillary action, with water being a notable example due to its hydrogen bonding.
- 📈 Specific heat and latent heat are two concepts used to quantify the energy changes during phase transitions, with specific heat relating to temperature change and latent heat to the phase change itself.
- 📊 A phase diagram is a graphical representation that shows the conditions under which different phases of matter are favored, with specific points like the triple point and critical point marking unique phase behaviors.
- 🌌 The ideal gas law (PV=nRT) and kinetic molecular theory (KMT) are foundational concepts for understanding the behavior of gases, with assumptions including elastic collisions, negligible intermolecular forces, and the gas filling its container.
- 💧 Solutions are homogeneous mixtures with varying concentrations measured by molarity (moles of solute/liters of solution) or molality (moles of solute/kg of solvent), and their properties are influenced by solute-solvent and solvent-solvent interactions.
- 📸 The photoelectric effect demonstrates the particle nature of light, where photons with energy above a threshold can eject electrons from a material, with the kinetic energy of the ejected electrons depending on the photon's energy minus the material's work function.
Q & A
What are intermolecular forces?
-Intermolecular forces are the forces of attraction or repulsion which act between neighboring particles (atoms, molecules, or ions). They are not as strong as the forces which hold the particles within a molecule (intramolecular forces).
What is the weakest intermolecular force and how does it work?
-The weakest intermolecular force is the London dispersion force. It occurs in all molecules and arises from the temporary polarization caused by the random movement of electrons, leading to an instantaneous dipole that induces a dipolar response in neighboring molecules.
How do dipole-dipole forces differ from London dispersion forces?
-Dipole-dipole forces are stronger than London dispersion forces. They occur between polar molecules where there is a permanent difference in electronegativity, resulting in a permanent dipole. This leads to a continuous attractive force between the positive end of one molecule and the negative end of another.
What is a hydrogen bond and why is it significant?
-A hydrogen bond is a special type of dipole-dipole interaction that occurs when hydrogen is bonded to a highly electronegative atom (like nitrogen, oxygen, or fluorine). It is significant because it is a particularly strong intermolecular force, although it is only present in molecules with specific types of bonds (F-H, O-H, or N-H).
Why are intermolecular forces important in phase changes?
-Intermolecular forces are crucial in phase changes because they determine the energy required to change a substance from one state of matter to another. Overcoming these forces is necessary to transition from a solid to a liquid (melting), from a liquid to a gas (evaporation or boiling), and vice versa.
What is the relationship between temperature and the phase of a substance?
-Temperature is a measure of the average kinetic energy of the particles in a substance. As you add energy to a substance, the particles move faster, and the temperature increases until a phase change occurs. During a phase change, the added energy goes into breaking intermolecular forces, causing a plateau in temperature until the phase change is complete.
What are the different types of phase changes and their corresponding terms?
-The different types of phase changes include: solid to liquid (melting), liquid to gas (evaporation or boiling), gas to liquid (condensation), liquid to solid (freezing), gas to solid (deposition), and solid to gas (sublimation).
What is the ideal gas law and how is it derived?
-The ideal gas law is given by the equation PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin. It is derived from the kinetic molecular theory, which assumes that gas particles are in constant random motion, collide elastically, are far apart relative to their size, and have no intermolecular forces.
How does the kinetic molecular theory (KMT) explain the behavior of gases?
-KMT explains that gas behavior is due to the motion of its particles. It states that gas particles move rapidly in straight lines and their collisions with each other and the container walls are elastic. The pressure exerted by a gas is due to the frequent collisions of the gas particles with the container walls.
What is the concept of partial pressure in the context of gas mixtures?
-Partial pressure refers to the pressure exerted by an individual gas in a mixture of non-reacting gases. The total pressure of the gas mixture is the sum of the partial pressures of the individual gases present in the mixture.
What are colligative properties and how do they relate to molality in solutions?
-Colligative properties are properties of a solution that depend only on the ratio of the number of solute particles to the number of solvent molecules, not on the nature of the chemical species in the solution. These include boiling point elevation, freezing point depression, and osmotic pressure. Molality is relevant for colligative properties because it represents the moles of solute per kilogram of solvent, which influences these properties.
What is the photoelectric effect and how is it quantified?
-The photoelectric effect is the emission of electrons from a material (usually a metal) when it is exposed to light. The kinetic energy of the emitted electrons can be quantified by the equation KE = hf - Φ, where KE is the kinetic energy of the emitted electrons, hf is the energy of the incident photon (h is Planck's constant and f is the frequency of the light), and Φ (phi) is the work function of the material, which is the minimum energy required to remove an electron from the surface.
Outlines
🔬 Introduction to Unit 3: Intermolecular Forces
The video begins with the host, Cara, introducing the topic of intermolecular forces, a part of 'baby chemistry'. She discusses her approach to covering the material, considering whether to proceed with units five, six, and seven or to focus on unit six and seven, and possibly create a practice test. Cara emphasizes the importance of understanding intermolecular forces, distinguishing them from intramolecular forces, and categorizes them from weakest to strongest: London dispersion forces, dipole-dipole forces, and hydrogen bonds. She uses the example of a sodium atom to explain London dispersion forces and highlights their presence in all molecules. The video promises an in-depth exploration of these forces and their significance in phase changes and other phenomena.
🌡️ Phase Changes and Intermolecular Forces
This paragraph delves into the relevance of intermolecular forces in phase changes, such as melting, evaporation, condensation, and sublimation. Cara explains how the strength of these forces affects the energy required to transition between the phases of matter. She also touches on concepts like surface tension and capillary action, which are influenced by intermolecular forces. The discussion continues with an introduction to phase diagrams, illustrating how temperature and energy input relate to phase transitions. Specific terms like latent heat, heat of fusion, and heat of vaporization are defined, and examples are provided to demonstrate their calculation and application.
📈 Understanding Phase Diagrams and Gas Laws
The host explains phase diagrams, focusing on the unique properties of water and the concept of density in different phases. Key points such as the triple point and critical point are highlighted. Cara then transitions into a discussion about gases, specifically the ideal gas law (PV=NRT) and its implications according to kinetic molecular theory (KMT). She describes how gas behavior can be predicted based on volume, pressure, and temperature, and introduces the concept of partial pressure. The paragraph concludes with an explanation of vapor pressure and its role in gas collection over water.
🌟 The Photoelectric Effect and Quantum Mechanics
Cara concludes the video with an overview of the photoelectric effect, a quantum mechanical phenomenon where light (photons) interacts with matter (atoms), leading to the emission of electrons. She discusses the relationship between the energy of photons, their frequency, and the work function required to release electrons from atoms. The host emphasizes that the energy of an ejected electron is dependent on the photon's energy minus the work function. The video wraps up with a reminder that the photon's energy is determined by its frequency or wavelength, not its intensity, which only affects the number of ejected electrons.
Mindmap
Keywords
💡Intermolecular forces
💡London dispersion forces
💡Dipole-dipole forces
💡Hydrogen bonding
💡Phase changes
💡Surface tension
💡Ideal gas law
💡Kinetic molecular theory (KMT)
💡Vapor pressure
💡Maxwell-Boltzmann distribution
💡Photoelectric effect
Highlights
Intermolecular forces are categorized from weakest to strongest as London dispersion forces, dipole-dipole forces, and hydrogen bonds.
London dispersion forces occur in all molecules and are caused by temporary polarization due to random electron movement.
Dipole-dipole forces are stronger and occur in polar molecules, resulting from the attraction between the positive end of one molecule and the negative end of another.
Hydrogen bonds are a special type of dipole-dipole interaction with a significant electronegativity difference, typically involving hydrogen, nitrogen, oxygen, or fluorine.
Intermolecular forces play a crucial role in phase changes, affecting how molecules transition from solid to liquid to gas.
Phase change processes include melting, evaporation, condensation, freezing, deposition, and sublimation.
A phase change diagram (specifically a pressure-temperature or PT diagram) illustrates the conditions under which phase transitions occur.
The ideal gas law, PV=nRT, is a fundamental principle in understanding the behavior of gases under various conditions.
Kinetic molecular theory (KMT) explains the behavior of gases based on assumptions such as elastic collisions, negligible intermolecular forces, and the gas filling the entire volume it occupies.
Partial pressure is the individual pressure exerted by each gas in a mixture and is additive to give the total pressure.
Vapor pressure is the pressure exerted by a vapor in equilibrium with its condensed phases at a given temperature.
Maxwell-Boltzmann distribution describes the distribution of molecular speeds in a gas, showing how the number of molecules varies with kinetic energy at a given temperature.
Molarity, molality, and normality are different types of concentrations used to describe solutions, each relevant for different chemical properties and reactions.
Colligative properties of solutions, such as boiling point elevation and freezing point depression, depend on the concentration of solute particles, not the nature of the particles.
The photoelectric effect demonstrates that light can be thought of as consisting of particles (photons), which have discrete energy levels.
The energy of a photon is determined by its frequency or inversely by its wavelength, not by the intensity of the light.
The work function is the minimum energy required to remove an electron from a material, and it is a key concept in the photoelectric effect.
Transcripts
Browse More Related Video

10.1 Intermolecular Forces | High School Chemistry

Van der Waals forces | States of matter and intermolecular forces | Chemistry | Khan Academy

AP Chem - Unit 3 Review - Intermolecular Forces & Properties

1.6 Intermolecular Forces | Organic Chemistry

11.1 Intermolecular Forces | General Chemistry
AP Chemistry Unit 2 Review
5.0 / 5 (0 votes)
Thanks for rating: