AP® Chemistry Weak Acid Equilibrium Questions
TLDRThe video script presents a comprehensive guide to solving an AP Chemistry free response question involving formic acid equilibrium. It begins by introducing the concept of weak acids and their equilibrium with reactants and products, emphasizing the importance of understanding the Ka expression for formic acid. The script then delves into calculating the pH of a 0.12 molar aqueous solution of formic acid, utilizing an ICE table and the Ka expression to find the concentration of H+ ions at equilibrium. A shortcut is employed by assuming the Ka value is small, simplifying the quadratic equation to a solvable form and ultimately yielding a pH of 2.3. The final part of the script addresses creating a buffer solution by adding sodium formate to formic acid, aiming for a pH of 3.90. The Henderson-Hasselbalch equation is applied to determine the required moles of sodium formate, with the pKa calculated from the Ka value. The process involves converting the problem into a logarithmic form and solving for the concentration of the formate ion, which leads to calculating the moles of sodium formate needed to achieve the desired pH. The script concludes with advice on practicing various example problems to recognize patterns and prepare effectively for the AP Chemistry exam.
Takeaways
- 🧪 The video provides a detailed walkthrough of an AP Chemistry free response question on weak acid equilibrium, specifically focusing on formic acid.
- 📝 It starts by explaining how to write the equilibrium constant expression (Ka) for formic acid, noting that water (H2O) does not appear in the expression.
- 🔬 Emphasizes the use of an ICE table (Initial, Change, Equilibrium) to determine the concentrations of chemical species at equilibrium for calculating pH.
- 📊 Demonstrates how to calculate the pH of a 0.12 molar formic acid solution using the ICE table and the assumption that changes in concentration due to dissociation are negligible.
- 🧐 Highlights a common shortcut in solving equilibrium problems by neglecting the change in initial concentration due to its small size relative to the dissociation constant (Ka).
- 📚 Explains the Henderson-Hasselbalch equation for creating a buffer solution with sodium formate, detailing how to calculate the necessary concentration of sodium formate for a desired pH.
- 🧩 Provides a step-by-step guide on manipulating the Henderson-Hasselbalch equation to find the required amount of sodium formate to add to a formic acid solution to achieve a specific buffer pH.
- 📉 Discusses the relationship between hydronium (H3O+) and hydrogen ions (H+), explaining that they are interchangeable and commonly used in acid-base equations.
- 💡 Advises on the importance of making and justifying assumptions in calculations during exams to aid understanding and ensure accuracy when simplifying complex problems.
- 🎓 Encourages extensive practice with AP Chemistry problems to recognize patterns and improve problem-solving skills for the exam.
Q & A
What is the main topic of the video?
-The video discusses a weak acid equilibrium question, specifically an AP Chemistry free response question involving formic acid.
Why is formic acid considered a weak acid?
-Formic acid is considered a weak acid because it does not fully ionize in water, and its products and reactants are in equilibrium with each other.
What is the equilibrium constant expression for formic acid?
-The equilibrium constant expression, or Ka, for formic acid is the concentration of hydronium (H+) times the concentration of the formate ion (A-), divided by the concentration of formic acid (HA).
Why is the hydronium ion (H3O+) often represented as H+ in the context of this video?
-H3O+ is often represented as H+ because they are different ways of expressing the same thing. The video emphasizes that both notations are used interchangeably on the AP exam.
What is the purpose of using an ICE table in the context of this problem?
-An ICE table (Initial, Change, Equilibrium) is used to determine the concentrations of chemical species at equilibrium, which is necessary for solving for the concentration of H+ to calculate pH.
How is the pH of a formic acid solution calculated?
-The pH of a formic acid solution is calculated by first determining the concentration of H+ ions at equilibrium using an ICE table and the Ka expression, and then applying the pH formula: pH = -log[H+].
What is the significance of the Henderson-Hasselbalch equation in buffer solutions?
-The Henderson-Hasselbalch equation is used to calculate the pH of a buffer solution. It relates the pH to the pKa of the acid, and the ratio of the concentrations of the conjugate base to the acid.
Why can the value of x be considered negligible in the calculation?
-The value of x can be considered negligible because Ka is a very small number, implying that the change in concentration (x) is much smaller than the initial concentration of the formic acid (0.15 M).
What is the assumption made in the calculation of x, and why is it important to note this?
-The assumption is that x is negligible compared to the initial concentration of formic acid. It's important to note this assumption to ensure transparency in the problem-solving process and to help the grader understand the reasoning behind the simplification.
How many moles of sodium formate are needed to create a buffer solution with a pH of 3.90 from a 250 mL of 0.10 M formic acid solution?
-To find the moles of sodium formate needed, first calculate the concentration of formate ion using the Henderson-Hasselbalch equation, then use the molarity equation (moles = molarity × liters) to find the moles required for the given volume.
Why is it important to show all work and explain thinking when solving multi-step AP Chemistry problems?
-Showing all work and explaining thinking is crucial for clear communication of the problem-solving process. It helps the grader understand the reasoning behind each step and ensures that partial credit is awarded for correct reasoning, even if a final answer is not reached.
What is the general strategy for approaching AP Chemistry free response questions?
-The general strategy includes understanding the different parts of the question, recognizing that parts may depend on each other, using provided formulas and constants, applying appropriate approximations, and practicing with various example problems to identify patterns and question types.
Outlines
🌟 Introduction to Weak Acid Equilibrium in AP Chemistry
The video begins by introducing a weak acid equilibrium question, which is a type of free response question in AP Chemistry. The presenter emphasizes the importance of preparation and provides a resource link for additional AP Chemistry materials. The focus is on formic acid, which is a weak acid that partially ionizes in water, leading to an equilibrium state between reactants and products. The video is structured into three parts, with each part potentially building upon the previous one. The first part involves writing the equilibrium constant expression (Ka) for formic acid, which is a fundamental step in solving acid equilibrium problems. The presenter explains that water, being a liquid, does not appear in the equilibrium expression and that the Ka formula is provided on the AP exam's equations page. The distinction between H+ and H3O+ (hydronium ion) is clarified, noting that they are interchangeable in this context.
📚 Calculating pH of a Formic Acid Solution
The second paragraph delves into calculating the pH of a 0.12 molar aqueous solution of formic acid. The presenter explains that pH is the negative log of the hydrogen ion (H+) concentration and introduces the use of an ICE table (Initial, Change, Equilibrium) to determine the concentrations of chemical species at equilibrium. It is noted that formic acid, being a weak acid, does not fully ionize, and only a portion of it will produce H+ ions. The ICE table is used to track the changes in concentrations as the reaction reaches equilibrium. The presenter also discusses a shortcut for solving the equilibrium expression due to the small value of Ka, which simplifies the calculation of x, representing the change in concentration of H+ ions at equilibrium. The calculation results in a pH value of 2.3 for the formic acid solution.
🧪 Creating a Buffer Solution with Sodium Formate
The third paragraph addresses the creation of a buffer solution by adding sodium formate to formic acid. The goal is to achieve a buffer with a pH of 3.90. The presenter outlines the use of the Henderson-Hasselbalch equation, which is provided on the AP Chemistry formula sheet, to solve for the concentration of the formate ion (A-) needed to create the buffer. The equation is rearranged to solve for the formate concentration, and the presenter demonstrates how to calculate the pKa from the given Ka value. After determining the concentration of formate required, the presenter uses the molarity equation to calculate the number of moles of sodium formate that must be added to 250 milliliters of a 0.10 molar formic acid solution to achieve the desired pH. The presenter encourages viewers to show their work and think through each step, even if they make mistakes in earlier parts of the problem.
🎓 Conclusion and Advice for AP Chemistry Test Takers
In the final paragraph, the presenter concludes with advice for students preparing for the AP Chemistry exam. They stress the importance of practicing various example problems to recognize patterns and become familiar with the types of questions commonly asked. The presenter encourages students to continue working through problems, showing their work and detailing their thinking, which will ultimately help them feel more comfortable and prepared for the test. The video ends with a wish for good luck to all students taking the AP Chemistry exam.
Mindmap
Keywords
💡Weak Acid
💡Equilibrium Constant (Ka)
💡Ionization
💡Hydronium Ion (H3O+)
💡pH
💡ICE Table
💡Buffer Solution
💡Henderson-Hasselbalch Equation
💡pKa
💡Molarity
💡Significant Figures
Highlights
Formic acid reacts with water according to the ionization equation provided, and we use the Ka (acid dissociation constant) to understand this reaction.
Formic acid is a weak acid that doesn't fully ionize. Instead, the products and reactants are in equilibrium.
The equilibrium expression for formic acid is derived from the concentrations of its ionized and non-ionized forms.
H2O is a liquid and doesn't appear in the equilibrium expression, while the hydronium ion (H3O+) and hydrogen ion (H+) are essentially the same thing.
An ICE (Initial, Change, Equilibrium) table is used to determine concentrations at equilibrium, crucial for solving weak acid problems.
The change in formic acid's concentration at equilibrium is assumed to be a small value (x) due to its weak acid nature.
The equation is solved using approximations, ignoring x when subtracted from the initial concentration due to the small Ka.
The pH is determined from the concentration of H+, calculated by solving the equation involving Ka and equilibrium concentrations.
To create a buffer solution with sodium formate, the Henderson-Hasselbalch equation is used.
In the Henderson-Hasselbalch equation, pH equals pKa plus the logarithm of the ratio between the conjugate base and the weak acid concentrations.
Formate ion (conjugate base) concentration is determined from the solution's volume and desired pH.
Molarity equals moles divided by liters, and rearranging this formula allows for calculating the necessary moles of sodium formate to add.
Always show your work and detail your reasoning to ensure clear understanding, especially when assumptions are made for approximations.
AP exam questions often contain interconnected sections, but not all parts depend on previous ones.
Working through numerous problems reveals patterns and prepares students for frequently asked question types.
Transcripts
Browse More Related Video

Calculate Ka from pH ICE Table

Acids and Bases: Buffer Calculation - Past Paper Exam Question Walkthrough|AQA A Level Chemistry

Calculating the pH of Weak Acids

Calculate the pH of 0.1 M Acetic Acid
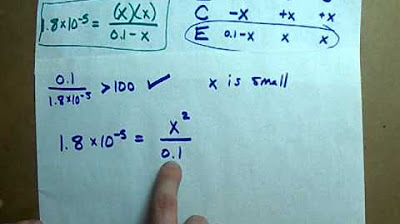
pH of a Weak Acid (0.1 M Acetic Acid) EXAMPLE

Find the Ka of an acid (Given pH) (0.1 M Hypochlorous acid) EXAMPLE
5.0 / 5 (0 votes)
Thanks for rating: