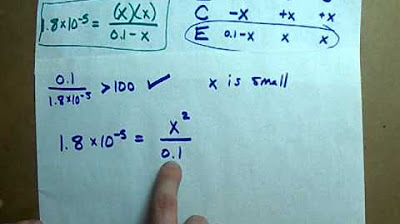Find the Ka of an acid (Given pH) (0.1 M Hypochlorous acid) EXAMPLE
TLDRThe video script explains the process of calculating the equilibrium constant (KA) for a weak acid, using the example of HClO. It emphasizes understanding the dissociation of the acid into H+ and ClO- ions and using the given pH to determine the concentration of H+. The script then illustrates how to set up an ICE (Initial, Change, Equilibrium) table and solve for the unknown concentration (X), which represents the concentration of H+. Finally, it demonstrates how to use the concentrations at equilibrium to calculate KA, resulting in a legitimate value for a weak acid.
Takeaways
- 📚 KA stands for the equilibrium constant for the dissociation of an acid.
- 🧪 When given pH and concentration, the goal is to calculate the acid's KA value.
- 🌟 HClO dissociates in water to form H+ and ClO- ions, with hypochlorite being the anion.
- 📈 The equilibrium expression for the reaction involves concentrations of products and reactants at equilibrium.
- 🔍 An ICE (Initial, Change, Equilibrium) table helps to set up the reaction and find the concentration of each species.
- 💡 The concentration of H+ (X) is found by using the formula 10^(-pH), in this case, 10^(-4.23).
- 🔧 The concentration of H+ (X) is equal to the concentration of ClO- (X) at equilibrium.
- 📊 The concentration of HClO at equilibrium is the initial concentration minus the change (X).
- 🧠 The KA value is calculated by the product of the concentrations of the ions (H+ and ClO-) divided by the concentration of the undissociated acid (HClO).
- 🔢 The calculated KA value of 3.47 × 10^(-8) is a legitimate value for a weak acid.
- 🎓 This method is an effective way to calculate the KA of a weak acid when given the pH and concentration.
Q & A
What does KA stand for in the context of chemistry?
-KA stands for the equilibrium constant for the dissociation of an acid. It is a measure of the strength of an acid, indicating how readily the acid donates protons (H+ ions) in solution.
What is the relationship between pH and H+ ion concentration?
-The pH of a solution is the negative logarithm (base 10) of the concentration of H+ ions. The formula is pH = -log[H+]. A lower pH value indicates a higher concentration of H+ ions, meaning the solution is more acidic.
How is the dissociation of HCl represented in an aqueous solution?
-HCl is a strong electrolyte and dissociates completely in water to form H+ and Cl- ions. The equation for this dissociation is: HCl → H+ + Cl-. Since it is a strong acid, the reaction is not reversible and does not use a reversible arrow.
What is the hypochlorite ion?
-The hypochlorite ion is ClO-. However, in the context of the script, it seems there was a confusion between chloride (Cl-) and hypochlorite (ClO-). The chloride ion is the conjugate base formed when a strong acid like HCl dissociates in solution.
How do you calculate the concentration of H+ ions given the pH value?
-Given the pH value, you can calculate the concentration of H+ ions using the formula: [H+] = 10^(-pH). For example, if the pH is 4.23, the concentration of H+ ions would be 10^(-4.23) M.
What is an ICE table and how is it used in calculating KA?
-An ICE table, which stands for Initial, Change, and Equilibrium, is a method used to keep track of the concentrations of reactants and products at different stages of a chemical equilibrium. It helps in setting up the equilibrium expression and solving for the unknown variables, such as the equilibrium constant (KA).
Why is it important to know the value of KA for an acid?
-Knowing the value of KA for an acid is important because it provides a quantitative measure of the acid's strength. A larger KA value indicates a stronger acid, which means it dissociates more completely in solution. This information is crucial in various chemical calculations and understanding reaction dynamics.
How does the concentration of HCl initially and at equilibrium relate to the calculation of KA?
-In the calculation of KA, the initial concentration of HCl is usually given, and the change in concentration represents the amount of HCl that has dissociated. At equilibrium, the concentration of HCl will be the initial concentration minus the change (X). These values are used in the equilibrium expression to solve for KA.
What is the significance of the equilibrium constant (KA) in predicting the behavior of an acid in solution?
-The equilibrium constant (KA) is significant as it predicts the extent to which an acid will dissociate in solution. A large KA value indicates that the acid dissociates largely, forming more products at equilibrium, which means the acid is strong. Conversely, a small KA value suggests limited dissociation, indicating a weak acid.
How does the concentration of H+ ions affect the pH value of a solution?
-The concentration of H+ ions directly affects the pH value of a solution. As the concentration of H+ increases, the pH value decreases, making the solution more acidic. Conversely, a lower concentration of H+ ions results in a higher pH value, indicating a less acidic (more basic) solution.
What is the role of the calculator in determining the concentration of H+ ions from the pH value?
-A calculator is used to compute the antilog (the number obtained by taking the logarithm to the base 10) of the negative pH value to find the concentration of H+ ions. For example, for a pH of 4.23, you would calculate 10^(-4.23) to find the H+ ion concentration.
What is the process of calculating the KA of an acid from a given pH and concentration?
-To calculate the KA of an acid from a given pH and concentration, you first determine the concentration of H+ ions using the pH value (using the formula [H+] = 10^(-pH)). Then, using an ICE table, you set up the equilibrium expression for the dissociation of the acid. By solving for the unknown concentration variables (X), you can calculate the ratio of the product concentrations to the reactant concentration at equilibrium, which gives you the value of KA.
Outlines
📚 Calculating the Acid Dissociation Constant (Ka)
This paragraph delves into the process of calculating the Ka of an acid when given its pH at a specific concentration. It begins with a brief discussion on the meaning of Ka, which is the equilibrium constant for the dissociation of an acid. The example of HClO dissociating into H+ and ClO- ions is used to illustrate the concept. The paragraph then explains how to use an ice table to determine the concentration of each species at equilibrium, highlighting the importance of understanding the dissociation process. It proceeds to calculate the concentration of H+ using the given pH value, and subsequently uses this information to find the Ka value. The explanation is thorough, walking through each step of the calculation and emphasizing the relevance of understanding these chemical concepts.
Mindmap
Keywords
💡KA
💡pH
💡Hypochlorite ion
💡Equilibrium expression
💡Concentration
💡ICE table
💡Dissociation
💡Weak acid
💡Equilibrium
💡10 to the power of negative
💡Chemical reaction
Highlights
Calculating the KA of an acid using given pH and concentration
Understanding the meaning of KA as the equilibrium constant for the dissociation of an acid
Dissociation of HClO in solution into H+ and ClO- ions
Establishing the equilibrium expression for the reaction
Using an ICE (Initial, Change, Equilibrium) table to determine concentrations at equilibrium
Setting up the equilibrium concentrations with HClO initially at 0.1 and the other concentrations as zero
Finding the concentration of H+ using the given pH value
Calculating the concentration of H+ as 10 to the power of negative pH
Deducing that the concentration of H+ (X) is 5.89 times 10 to the negative 5
Expressing the relationship between the concentrations of H+, ClO-, and HClO at equilibrium
Recognizing that the concentration of HClO is negligible compared to 0.1
Calculating the KA value using the known concentrations and the equilibrium expression
Arriving at a KA value of 3.47 times 10 to the negative 8
The method provides a legitimate value for the KA of a weak acid
The process demonstrates a comprehensive approach to solving chemical equilibrium problems
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: