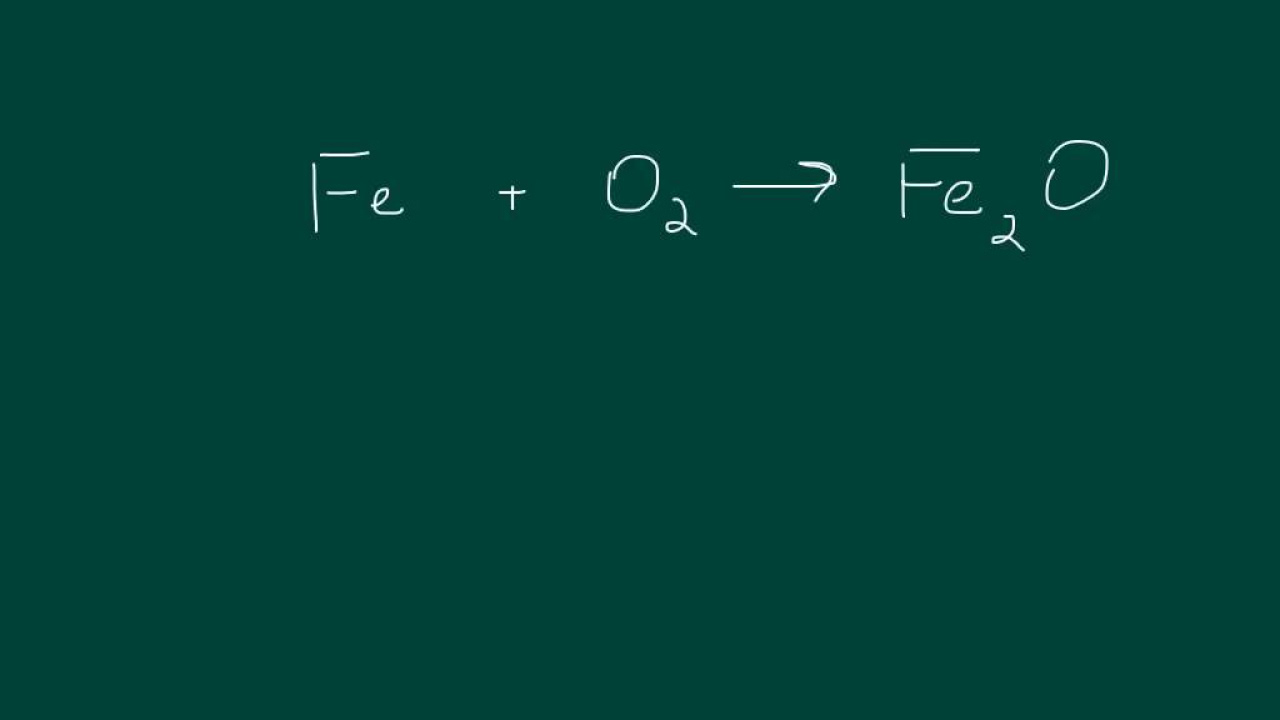Balancing Equations: ABCD Method
TLDRThe video script introduces the ABCD method for balancing chemical equations, a mathematical approach that serves as an alternative to the traditional hit-and-trial or table method. The ABCD method involves assigning coefficients (a, b, c, d) to each compound in the equation and forming equations based on the number of atoms of each element. The key is to identify the coefficient that occurs most frequently and set it to 1, then solve for the remaining coefficients. The method simplifies the balancing process by transforming it into a mathematical problem, which can handle even complex equations. The script emphasizes the importance of careful counting and solving linear equations, and it concludes with the recommendation to practice patience and to always double-check the balanced equation for accuracy. The video also encourages students to explore other courses offered on the website, including subjects for different educational boards and programming languages.
Takeaways
- 🔍 **Understanding the ABCD Method**: The ABCD method is a systematic approach to balance chemical equations using coefficients (a, b, c, d...) and is particularly useful when the trial-and-error method fails.
- 📚 **Applying Coefficients**: Start by assigning coefficients (a, b, c, d...) to each compound in the chemical equation, which act as variables that you will solve for.
- ⚖️ **Balancing Each Element**: Create equations for each element present in the reaction, ensuring that the number of atoms for each element is equal on both sides of the equation.
- 🔢 **Solving for Coefficients**: Determine which coefficient appears most frequently and set it to 1 to simplify calculations. Then, solve for the remaining coefficients.
- 📨 **Eliminating Fractions**: If the solution results in fractions, multiply all coefficients by the least common multiple (LCM) of the denominators to eliminate them.
- 🔬 **Practical Application**: The ABCD method is demonstrated through practical examples, showing how to balance complex chemical equations step by step.
- 📈 **Choosing the Right Method**: The instructor recommends starting with the table method for understanding, then moving to the hit-and-trial method, and finally using the ABCD method as a backup when other methods fail.
- 🤓 **Patience and Practice**: Balancing chemical equations requires patience and practice, much like solving a Rubik's Cube, emphasizing the importance of persistence.
- 📝 **Checking Your Work**: After balancing, verify that the number of atoms for each element is equal on both sides and that there are no common factors that could simplify the coefficients further.
- 🚫 **Avoiding Unbalanced Equations**: Writing unbalanced equations can lead to a zero score, so it's crucial to ensure that the balanced equation is accurate before submission.
- 🌐 **Additional Resources**: The speaker encourages students to explore other courses offered on their website, including subjects like physics, chemistry, biology, and math, as well as programming languages like Java and Python.
Q & A
What is the ABCD method for balancing chemical equations?
-The ABCD method is a systematic approach to balancing chemical equations by using coefficients (a, b, c, d, etc.) and turning the process into a mathematical problem. It involves setting up equations for each element in the reaction to ensure that the number of atoms for each element is equal on both sides of the equation.
Why is the ABCD method considered powerful for balancing chemical equations?
-The ABCD method is considered powerful because it can handle even complicated chemical equations. It uses mathematical logic to balance equations, which can be more efficient than trial and error methods, especially when the equation involves multiple compounds or is particularly complex.
How does the ABCD method turn the process of balancing chemical equations into a mathematical problem?
-The ABCD method involves assigning coefficients (a, b, c, d, etc.) to each compound in the chemical equation. Then, for each element present in the reaction, an equation is formed that equates the total count of atoms of that element on both sides of the equation, including the coefficients. Solving these equations yields the values of the coefficients, which are then used to balance the chemical equation.
What is the first step in applying the ABCD method?
-The first step in applying the ABCD method is to assign coefficients (a, b, c, d, etc.) to each compound in the chemical equation, starting from the reactants side.
How do you decide which coefficient to set to 1 during the ABCD method?
-You decide which coefficient to set to 1 by identifying the coefficient that occurs the maximum number of times in the equations you've formed. Setting this coefficient to 1 simplifies the calculations and makes it easier to solve for the other coefficients.
What is the purpose of setting a coefficient to 1 in the ABCD method?
-Setting a coefficient to 1 simplifies the system of equations that you need to solve. It turns one of the variables into a known quantity, which makes it easier to find the values of the other coefficients.
How do you handle fractions when using the ABCD method?
-Fractions can be eliminated by multiplying the entire equation by the least common multiple (LCM) of the denominators. This ensures that all the denominators cancel out, resulting in whole number coefficients.
Why is it important to check your work after balancing chemical equations with the ABCD method?
-Checking your work is crucial to ensure that the balanced chemical equation is correct. You should verify that the number of atoms for each element is equal on both sides of the equation and that there are no common factors that could simplify the coefficients further.
What does the instructor recommend for someone who is comfortable with balancing chemical equations?
-The instructor recommends starting with the hit and trial method for someone who is comfortable with balancing chemical equations, as it can be quicker and easier once you understand the process. If the hit and trial method is not working, then the ABCD method can be used as a backup plan.
Why is the table method mentioned in the script?
-The table method is mentioned as a way to understand the process of balancing chemical equations. It helps to visualize the count of atoms on both sides of the equation and is useful for beginners to grasp the concept of balancing before moving on to more advanced methods like hit and trial or ABCD.
What are some other courses offered by the academy mentioned in the script?
-The academy offers courses in physics, chemistry, biology, and maths for CBSE Class 8, 9, and 10. Additionally, they provide courses for the ICSE students and programming courses in Java and Python. They also offer physics and chemistry for the Cambridge IGCSE board.
Outlines
📚 Introduction to the ABCD Method for Balancing Chemical Equations
The video begins with a welcome message and an introduction to the ABCD method, a mathematical approach for balancing chemical equations. The instructor emphasizes the method's utility as an alternative to hit-and-trial or the table method, especially for complex equations. The video also promotes other courses offered by the academy, including subjects like physics, chemistry, biology, and math for various grade levels, as well as coding in Java and Python. The importance of balancing equations in adherence to the law of conservation of mass is mentioned, and the ABCD method is presented as a powerful tool for students to master.
🔍 Understanding the ABCD Method and Setting Up Coefficients
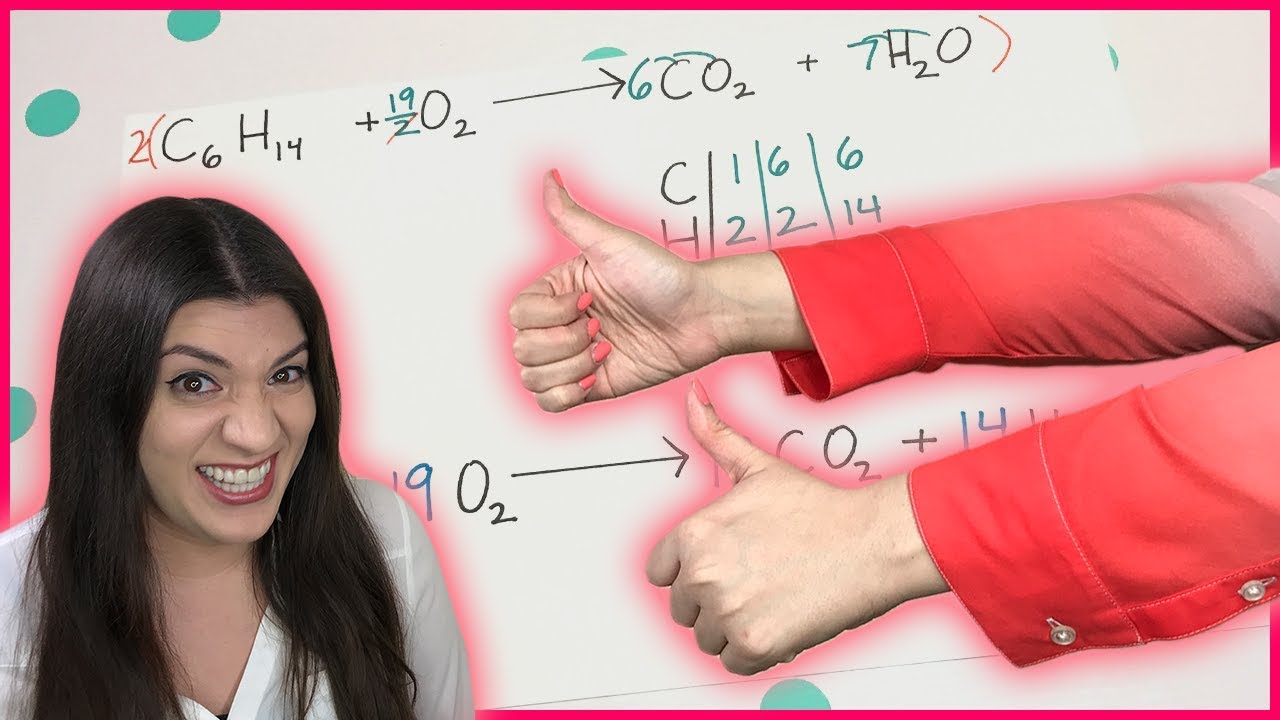
The paragraph explains the ABCD method in detail, starting with the placement of coefficients (a, b, c, d) for each substance in the chemical equation. The process involves creating equations based on the number of atoms for each element present, ensuring they are equal on both sides of the equation. The instructor advises setting the most frequently occurring coefficient to 1 to simplify calculations. The paragraph concludes with a practical example using ammonia and oxygen to demonstrate the method, emphasizing the need for careful counting to avoid errors.
🧮 Solving Coefficient Equations and Balancing the Equation
This section delves into solving the equations derived from the coefficients of the chemical equation. The process involves finding the values of coefficients a, b, c, and d by substitution and using the relationships between them. The instructor demonstrates solving for each coefficient step by step, transforming the chemistry problem into a mathematical one. The importance of eliminating fractions by multiplying with the least common multiple (LCM) of the denominators is highlighted to present a clean, balanced chemical equation. The paragraph concludes with a successful balance of the example equation, showcasing the effectiveness of the ABCD method.
📝 Applying the ABCD Method to a New Chemical Equation
The instructor continues with another example, emphasizing the method's application to balance more complex equations. The process involves identifying the elements involved, setting up equations based on the count of atoms for each element, and solving for the coefficients. The paragraph details the step-by-step approach, including the selection of the most frequently occurring coefficient to set as 1, solving the resulting equations, and adjusting for fractions by multiplying with the LCM of the denominators. The final balanced equation is presented, verifying its correctness through a check of the atom count for each element.
🔢 Balancing Equations Using the ABCD Method: A Step-by-Step Guide
This paragraph provides a comprehensive guide on using the ABCD method to balance chemical equations. It outlines the steps from identifying the elements in the equation to forming and solving equations based on the count of atoms for each element. The importance of careful counting and accurate equation formation is stressed. The paragraph also discusses the strategy of selecting the most frequently occurring coefficient and solving for the remaining coefficients, followed by substituting these values back into the equation and eliminating fractions. The process is illustrated with a detailed example, emphasizing the method's effectiveness and the need for meticulous practice.
🎓 Conclusion and Recommendations on Balancing Chemical Equations
The final paragraph wraps up the lesson by recommending the order in which students should approach balancing chemical equations. It suggests starting with the table method for a foundational understanding, followed by the hit-and-trial method for practice. If neither works, the ABCD method is presented as a reliable backup plan. The instructor advises against displaying the ABCD method in exams if not allowed, but encourages its use as a personal strategy for solving complex equations. The importance of checking balanced equations for accuracy and the absence of common multipliers is emphasized. The video concludes with a reminder of the importance of balancing equations and a prompt to explore other courses offered by the academy.
Mindmap
Keywords
💡ABCD method
💡Chemical equations
💡Coefficients
💡Law of conservation of mass
💡Hit-and-trial method
💡Table method
💡Linear equations
💡Substitution
💡Elimination
💡Least common multiple (LCM)
💡Manocha Academy
Highlights
Introduction to the ABCD method for balancing chemical equations, a powerful alternative to traditional methods.
The ABCD method uses mathematical tricks to balance any chemical equation, even complex ones.
Emphasis on learning the ABCD method as a backup plan when other methods fail.
Explanation of coefficients as multipliers in the context of the ABCD method.
Practical example demonstrating the step-by-step process of the ABCD method.
Technique of setting the most frequently occurring coefficient to 1 for easier calculation.
Clarification on how to handle fractions in coefficients by multiplying with the least common multiple (LCM).
The transformation of a chemistry problem into a mathematical one using the ABCD method.
Recommendation to practice the ABCD method for efficiency in balancing chemical equations.
Suggestion to use the table method initially for understanding and then move to the hit-and-trial method.
Advocacy for the ABCD method as a secret weapon for difficult equations when other methods are ineffective.
Importance of checking the balanced equation for accuracy and the absence of common factors.
The presenter's recommendation on which method to use based on the situation: table method for understanding, hit-and-trial for practice, and ABCD for complex equations.
Highlighting the necessity of balancing equations for academic integrity, as unbalanced equations can lead to zero marks.
Promotion of additional courses offered by the academy in physics, chemistry, biology, maths, and coding.
Encouragement for students to subscribe to the academy's YouTube channel and follow on social media for continuous learning.
Emphasis on the practical application of the ABCD method in solving real chemical equations, showcasing its effectiveness.
Advice for students to practice patience and diligence when using the ABCD method, comparing it to solving a Rubik's Cube.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: