Balancing Equations Made Easy
TLDRThis engaging chemistry class transcript focuses on the critical skill of balancing chemical equations, a fundamental concept that students must master. The instructor emphasizes the importance of this skill and aims to simplify the process for students by introducing various techniques, including the table method, hit and trial method, and the advanced ABCD method. The class not only covers the 'why' behind balancing equations, which is to adhere to the law of conservation of mass, but also provides practical steps to achieve balance without relying on the mass of elements. The instructor uses relatable analogies, such as a fruit seller adjusting weights, to explain the process. The transcript also touches on the use of fractions for more complex equations and stresses the importance of practice for mastering the skill. Additionally, the class promotes other courses offered by the academy in subjects like physics, biology, maths, and computer coding, encouraging continuous learning and skill development.
Takeaways
- 🧪 Balancing chemical equations is crucial and ensures the law of conservation of mass is satisfied in chemical reactions.
- 📊 The table method involves creating a table to count the atoms of each element and is a good starting point for beginners.
- ✅ The hit and trial method is a quicker approach where you adjust the coefficients by trial and error until the equation is balanced.
- 📝 You should not change compound formulas during the balancing process; only use multiplication to adjust coefficients.
- 🚫 Leaving fractions in the final answer is not acceptable in chemistry, so multiply the entire equation to eliminate any denominators.
- 🔢 It's important to check for common factors among the coefficients after balancing to ensure the simplest form of the equation.
- 📚 Practice is key to mastering the art of balancing chemical equations, which can be compared to solving a Rubik's Cube.
- 📈 Start with the table method, then progress to hit and trial, and for more complex equations, an advanced method called ABCD will be introduced.
- 🤔 When using fractions to balance equations, ensure to multiply the entire equation by the denominator to get rid of fractions.
- 📌 Always double-check your work by comparing the number of atoms of each element on both sides of the equation.
- 🌐 Manocha Academy offers a range of courses including physics, chemistry, biology, maths, and computer programming for various educational boards.
Q & A
Why is balancing chemical equations important?
-Balancing chemical equations is important because it ensures the law of conservation of mass is satisfied, meaning that the number of atoms of each element is the same on both sides of the equation, indicating that no atoms are lost or gained during a chemical reaction.
What is the basic principle behind the table method for balancing chemical equations?
-The table method involves creating a table that lists the number of atoms of each element on both sides of the equation. The goal is to adjust the coefficients in front of the chemical formulas until the number of atoms for each element is equal on both sides.
How does the hit and trial method differ from the table method?
-The hit and trial method involves adjusting the coefficients of the chemical equation without creating a table. It is a more mental approach where you try different numbers until the equation is balanced, starting with the elements that seem easiest to balance.
What is an improper fraction in the context of balancing chemical equations?
-An improper fraction is used when you multiply the coefficients of a chemical equation by a fraction to balance it more quickly. It is a number that represents a ratio greater than one, such as 3/2. However, the final answer should not contain fractions, and the equation should be multiplied by the denominator to eliminate the fraction.
Why should you not leave fractions in the final balanced chemical equation?
-Fractions should not be left in the final balanced chemical equation because they imply a portion of a molecule or atom, which is not practical in real chemical reactions. The coefficients in a balanced equation should be whole numbers.
What is the ABCD method mentioned in the script?
-The ABCD method is an advanced technique for balancing chemical equations that is particularly useful for more complex equations. It will be taught in the next class and is designed to help students solve difficult equations when other methods like hit and trial are not sufficient.
What should you do if you find that the coefficients in your balanced equation have a common factor?
-If the coefficients in your balanced equation have a common factor, you should divide each coefficient by the highest common factor to simplify the equation. This ensures that you have not multiplied unnecessarily and that your equation is in its simplest whole number form.
How can you check if your balanced chemical equation is correct?
-You can check if your balanced chemical equation is correct by comparing the number of atoms of each element on both sides of the equation. They should be equal. Additionally, ensure there are no common factors in the coefficients that could be further simplified.
What is the relationship between the atomic mass unit (AMU) and balancing chemical equations?
-The atomic mass unit (AMU) is a unit of mass that is used to express atomic and molecular masses. While balancing chemical equations, you are primarily ensuring the number of atoms of each element is equal on both sides, not the mass in AMU. However, the concept of AMU can help illustrate the principle of mass conservation in a chemical reaction.
Why is it important to practice balancing chemical equations?
-Practicing balancing chemical equations is important because it helps develop a deeper understanding of chemical reactions and the law of conservation of mass. It also improves problem-solving skills and the ability to manipulate chemical equations, which is a fundamental skill in chemistry.
What are some additional courses offered by the academy mentioned in the script?
-The academy offers courses in physics, chemistry, biology, and maths for CBSE Class 8, 9, and 10. They also provide courses for ICC students in the same subjects and classes, as well as Python Programming, Java coding, and physics and chemistry courses for the Cambridge IGCSE.
Outlines
👋 Introduction to Balancing Chemical Equations
The video begins with a welcome to a chemistry class focused on balancing chemical equations, emphasizing its importance and promising to simplify the process. The instructor plans to cover different techniques, including a recommended method for when one gets stuck. They also encourage students to check out other courses offered on their website, such as subjects for CBSE classes and programming languages, and to follow their social media for updates.
🔍 Understanding the Need for Balancing Equations
The paragraph explains the rationale behind balancing chemical equations by referencing the law of conservation of mass. It uses the example of a traditional beam balance to illustrate the concept of balancing masses, which is then translated into balancing the number of atoms in a chemical equation. The famous equation of hydrogen plus oxygen equals water is used to demonstrate the process of converting a word equation into a chemical equation and the importance of using molecular forms.
📊 The Table Method for Balancing Equations
The instructor introduces the table method, which involves creating a table to count the number of atoms of each element and then adjusting the multipliers to balance the counts. The method is demonstrated using the example of hydrogen and oxygen forming water. The importance of starting with the easiest element to balance and being frugal with multipliers is highlighted.
⚖️ Applying the Table Method to More Complex Equations
The table method is further practiced with more complex equations involving iron, hydrogen, oxygen, and other elements. The process involves identifying the elements, counting the atoms, and using multipliers to balance each row of the table. The goal is to balance each element one at a time, adjusting as necessary until the entire table is balanced.
🎯 Hit and Trial Method and Fractions in Balancing
The hit and trial method is introduced as a quicker approach to balancing equations without drawing a table. It involves mentally trying different multipliers and starting with the easiest elements. The instructor also demonstrates how to use fractions to balance equations more efficiently, especially when there's one element remaining, and emphasizes the need to eliminate fractions from the final answer by multiplying the entire equation by the denominator.
🤔 Dealing with Fractions and Final Checks
The paragraph discusses the use of fractions in balancing equations, showing how to multiply by the denominator to eliminate fractions from the final answer. It also emphasizes the importance of checking for common factors among the multipliers to ensure the most simplified form of the balanced equation. The instructor reminds students not to change compound formulas and to practice balancing equations using the table and hit and trial methods.
📚 Homework and Upcoming ABCD Method
The instructor assigns homework to practice the table and hit and trial methods and teases an upcoming lesson on the ABCD method for more complex equations. They remind students to be patient and practice regularly to improve their skills. The video concludes with a reminder to check out other courses and to follow their academy on social media for continuous learning.
Mindmap
Keywords
💡Balancing Chemical Equations
💡Law of Conservation of Mass
💡Diatomic Molecules
💡Table Method
💡Hit and Trial Method
💡ABCD Method
💡Fractions in Balancing
💡Common Factors
💡
💡Practice
💡Manocha Academy
💡Computer Programming
Highlights
The importance of balancing chemical equations is emphasized as a fundamental skill in chemistry.
Different techniques for balancing chemical equations are introduced, including the table method, hit and trial method, and the ABCD method.
The law of conservation of mass is explained as the reason for balancing chemical equations.
The concept of atomicity in elements and molecules is discussed to understand how to write chemical equations correctly.
A practical example of balancing the equation for the formation of water from hydrogen and oxygen is provided.
The table method is demonstrated step by step for balancing chemical equations.
The hit and trial method is introduced as a quicker way to balance equations without the need for a table.
An innovative approach using fractions to balance equations is presented, which can be faster for certain types of equations.
The necessity of not leaving fractions in the final answer of a balanced chemical equation is discussed.
The ABCD method is mentioned as an advanced technique suitable for more difficult equations, to be covered in a subsequent class.
Practical tips are given for checking the balance of an equation by comparing the number of atoms of each element.
The importance of not over-balancing equations and avoiding common multipliers is highlighted.
The class is encouraged to practice the table method and hit and trial method as homework before learning the ABCD method.
The presenter promotes other courses available on their website, including subjects like physics, biology, and mathematics.
The value of patience and practice in mastering the skill of balancing chemical equations is emphasized.
The presenter invites students to subscribe to their YouTube channel and follow on social media for continued learning.
An open invitation for doubts and questions is extended to ensure understanding of the material covered in the class.
Transcripts
Browse More Related Video
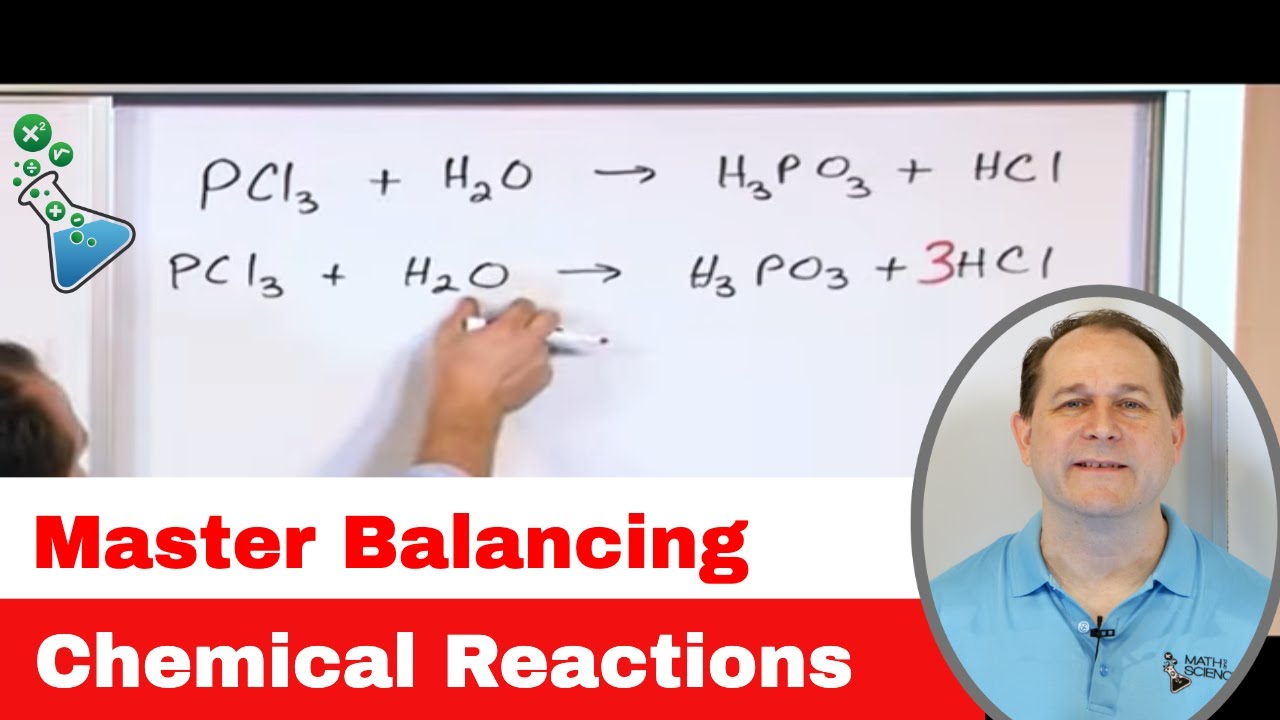
Mastering the Art of Balancing Chemical Reactions in Chemistry

Balancing Equations: ABCD Method

Equations of Motion Sums
Rearranging Equations - GCSE IGCSE 9-1 Physics - Science - Top Grade Top Up for GCSE and IGCSE

How to Balance Chemical Equations in 5 Easy Steps: Balancing Equations Tutorial
Center of Mass | Physics with Professor Matt Anderson | M12-04
5.0 / 5 (0 votes)
Thanks for rating: