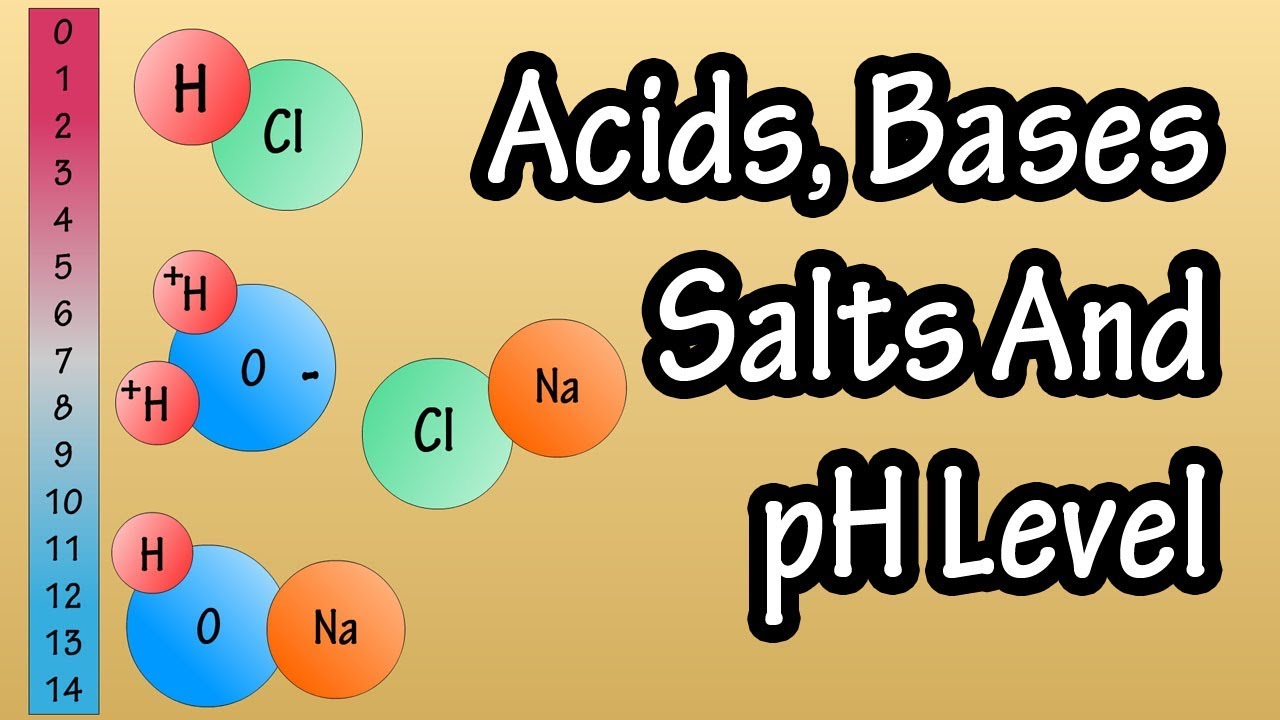
GCSE Chemistry - Acids and Bases #34
TLDRThis informative video delves into the pH scale, explaining its measurement from 0 to 14, with 7 representing neutrality. It highlights the role of acids and alkalis, using relatable examples like stomach acid and rainwater. The video also discusses various methods to measure pH, such as indicators and pH meters, and differentiates between acids, bases, and alkalis. It concludes with a mention of neutralization reactions and common examples of acids and bases, promoting a deeper understanding of these chemical concepts.
Takeaways
- 💧 The pH scale measures acidity or alkalinity of a solution on a scale from 0 to 14, with low numbers being acidic, high numbers being alkaline, and 7 being neutral.
- 🧠 Stomach acid has a pH around 2, showcasing a highly acidic environment, while acid rain is less acidic with a pH around 4.
- 📃 Alkalis like washing up liquid and bleach have higher pH values, around 9 and 12 respectively, indicating their basic nature.
- 🖌️ pH can be measured using indicators, which are chemical dyes changing color at different pH levels, or a pH probe for a more accurate, numeric reading.
- 🌊 Universal indicator is a popular choice for showing a wide pH range, changing colors from red (acidic) to blue/purple (alkaline).
- 💻 A pH probe provides precise measurements by electronically reading the pH level, reducing the need for subjective color interpretation.
- ⚗️ Acids are defined as substances that form solutions with a pH less than 7 and release hydrogen ions in water.
- 🧶 Bases have a pH greater than 7, while alkalis are water-soluble bases that form hydroxide ions in water.
- ▶ Acid and base reactions neutralize each other, producing a salt and water, with the pH of the resulting solution being neutral.
- 📚 It's useful to recognize common acids like hydrochloric, sulfuric, and nitric acid, and bases like sodium hydroxide or calcium carbonate.
Q & A
What is the pH scale and what does it measure?
-The pH scale is a measure of how acidic or alkaline a solution is, ranging from 0 to 14, with low numbers indicating acidity and high numbers indicating alkalinity. A pH of 7 is neutral, like pure water.
What is the pH of stomach acid and why is it important?
-The pH of stomach acid is around 2, which is highly acidic. This acidity is important for the digestion of food and for killing harmful bacteria.
How is acid rain characterized in terms of pH?
-Acid rain is characterized by having a pH around 4, which is more acidic than normal rainwater, hence its corrosive effects on the environment.
What is the function of washing up liquid in terms of pH?
-Washing up liquid has a pH of around 9, making it mildly alkaline. This property helps in breaking down and removing grease and dirt from dishes.
How does bleach differ from other alkalis in terms of pH?
-Bleach typically has a higher pH of around 12, making it a strong alkali. It is used for cleaning purposes, particularly in disinfecting and whitening.
What are the two methods of measuring pH mentioned in the script?
-The two methods of measuring pH are using indicators, which are chemical dyes that change color depending on the pH, and using a pH probe connected to a pH meter for a more accurate and precise electronic measurement.
What is a universal indicator and how does it work?
-A universal indicator is a wide-range indicator that contains a mixture of different dyes. It changes color across a wide range of pH values, from deep red in very acidic conditions to yellow-green and then bluey-purple as the pH increases towards alkalinity.
How can you define an acid according to the script?
-An acid can be defined as any substance that forms aqueous solutions with a pH less than 7. Acids release hydrogen ions in water, which makes the solution acidic.
What are the differences between bases and alkalis?
-Bases are substances with a pH greater than 7, while alkalis are a subgroup of bases that are soluble in water and form solutions with a pH greater than 7. Alkalis produce hydroxide ions (OH-) in water.
What is a neutralization reaction and what are its typical products?
-A neutralization reaction occurs when an acid reacts with a base to produce a salt and water. The reaction involves the combination of hydrogen ions (H+) from the acid and hydroxide ions (OH-) from the base to form H2O, resulting in a neutral pH of 7.
Name some common acids and bases mentioned in the script.
-Common acids include hydrochloric acid, sulfuric acid, and nitric acid. Common bases are often hydroxides or carbonates, like sodium hydroxide and calcium carbonate.
Outlines
📏 Understanding the pH Scale
This paragraph introduces the pH scale, a measure of how acidic or alkaline a solution is, ranging from 0 to 14. It explains that low pH values indicate high acidity, while high pH values indicate high alkalinity, with a pH of 7 being neutral, like pure water. Examples are given to illustrate the pH of various substances, such as stomach acid (pH ~2) and acid rain (pH ~4), and contrasted with household products like washing up liquid (pH ~9) and bleach (pH ~12). The paragraph emphasizes the importance of understanding pH and provides context for the subsequent discussion on acids and alkalis.
Mindmap
Keywords
💡pH scale
💡acids
💡alkalines
💡indicators
💡pH probe
💡neutralization reaction
💡hydrochloric acid
💡sodium hydroxide
💡sodium chloride
💡hydroxide ions
💡wide-range indicators
Highlights
The pH scale is a measure of how acidic or alkaline a solution is, ranging from 0 to 14.
Low pH numbers (below 7) indicate acidity, while high pH numbers (above 7) indicate alkalinity.
A neutral pH, like that of pure water, is 7, which is neither acidic nor alkaline.
Stomach acid has a pH of around 2, which helps to kill bacteria.
Acid rain has a pH of approximately 4, which is harmful to the environment.
Washing up liquid has a pH of around 9, showing its alkaline nature.
Bleach used for cleaning bathrooms has a pH of around 12, indicating a strong alkalinity.
pH can be measured using indicators, which are chemical dyes that change color depending on the pH.
Universal indicator is a common wide-range indicator that provides a color change across the pH scale.
A pH probe connected to a pH meter offers a more accurate and precise electronic measurement of pH.
Acids release hydrogen ions in water, resulting in a pH less than 7.
Bases, with a pH greater than 7, release hydroxide ions (OH-) in water.
Alkalis are a subgroup of bases that are soluble in water and have a pH greater than 7.
Neutralization reactions occur when an acid reacts with a base, producing salt and water.
The pH of the products of a neutralization reaction should be 7, indicating neutrality.
Common acids include hydrochloric acid, sulfuric acid, and nitric acid.
Common bases are often hydroxides or carbonates, like sodium hydroxide and calcium carbonate.
Understanding the pH scale and the properties of acids and bases is essential for various applications in everyday life.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: