Chapter 18: Example Unit Conversions with Light | CHM 214 | 152
TLDRThe video script discusses the properties of light, focusing on infrared spectroscopy to analyze functional groups in organic molecules. It illustrates how to calculate the wavelength, frequency, and energy (in joules per photon) of light at 3000 wave numbers, using the principles of wave properties and Planck's constant. The example demonstrates the conversion between different light properties and emphasizes the relatively low energy of a single photon compared to macroscopic scales.
Takeaways
- 🌟 Infrared spectroscopy is used to analyze functional groups in organic molecules, particularly the CH stretch region.
- 📐 The absorption of CH single bonds occurs at approximately 3,000 wave numbers.
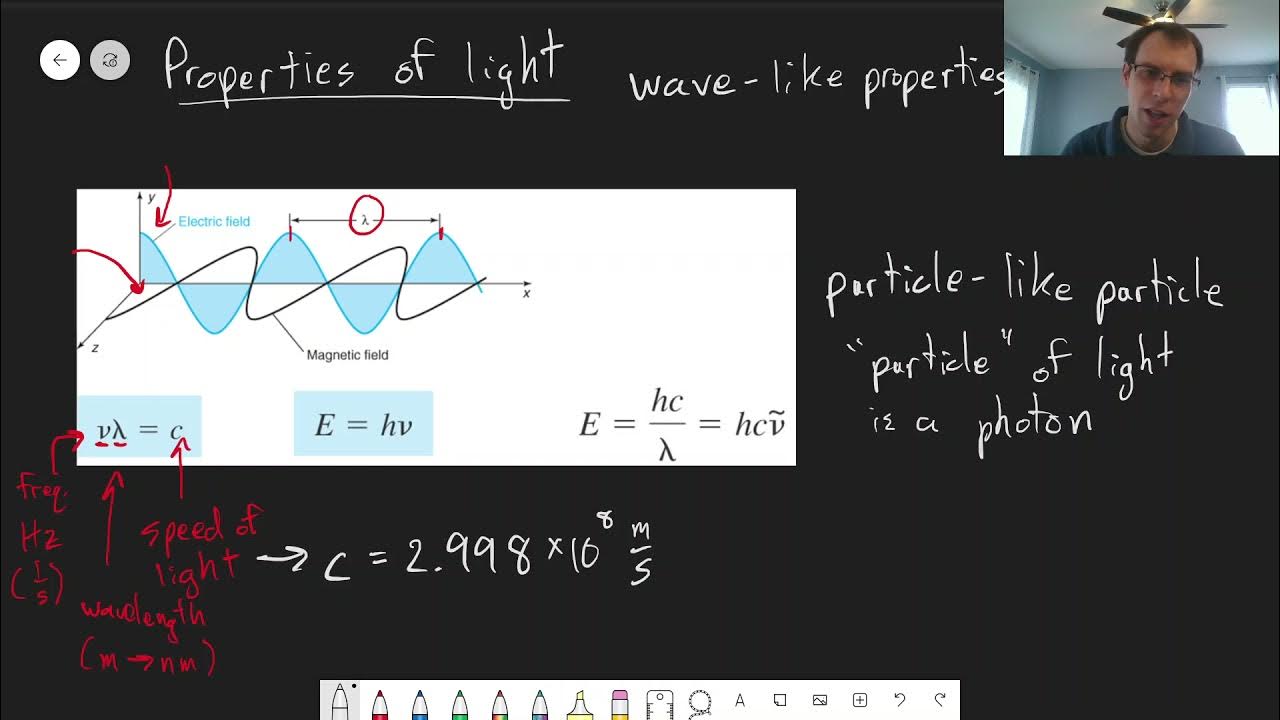
- 🔄 Wave number (cm⁻¹) can be converted to wavelength (cm) using the formula λ = 1/wave number.
- 📏 Wavelength is typically expressed in micrometers for infrared spectroscopy, with 3.33 μm corresponding to a 3,000 cm⁻¹ wave number.
- 🌐 The speed of light (c) is 2.998 x 10⁸ m/s, and can be used to calculate frequency (ν) using the equation ν = c/λ.
- 🖥️ When calculating frequency, it's important to ensure unit consistency, such as converting meters to centimeters if needed.
- 🎢 The frequency of light corresponding to a 3,000 cm⁻¹ wave number is 8.99 x 10¹³ Hz.
- 💡 Planck's constant (h) is 6.626 x 10⁻³⁴ Js and is used to calculate the energy (E) of a photon using the equation E = hν.
- 🌠 A single photon at 3,000 cm⁻¹ wave number has an energy of 5.96 x 10⁻²⁰ J, which is relatively low compared to macroscopic energy levels.
- 🔍 The process of converting between wavelength, frequency, wave number, and energy per photon is essential for understanding the properties of light.
- 🚀 This example illustrates the application of fundamental physics concepts in the study of light and its interaction with matter.
Q & A
What is the topic of discussion in the transcript?
-The topic of discussion is the properties of light, specifically focusing on an example calculation related to infrared spectroscopy and its application in identifying functional groups in organic molecules.
What is the significance of the CH stretch region in infrared spectroscopy?
-The CH stretch region is significant in infrared spectroscopy because it helps identify the presence of CH single bonds in organic molecules. An absorption at about 3000 wave numbers is indicative of this.
How is the wavelength related to the wave number?
-The wavelength is inversely related to the wave number. The wavelength can be calculated as one over the wave number, which gives the distance between the peaks of the wave.
What is the calculated wavelength for a 3000 wave number absorption?
-The calculated wavelength for a 3000 wave number absorption is 3.33 micrometers, after converting from centimeters to micrometers.
How can we find the frequency of light corresponding to a given wave number?
-The frequency of light can be found using the equation nu (frequency) equals c (speed of light) over lambda (wavelength) or c times the wave number. For the given wave number of 3000 cm^-1, the frequency is 8.99 x 10^13 Hz.
What is the energy of a single photon corresponding to a 3000 wave number absorption?
-The energy of a single photon corresponding to a 3000 wave number absorption is 5.96 x 10^-20 Joules. This is calculated using Planck's constant (h) times the frequency (nu).
How does the energy of a single photon of infrared light compare to that of visible light?
-The energy of a single photon of infrared light (around 10^-20 Joules) is less than that of visible light, which is around 10^-19 Joules. This difference reflects the fact that infrared light has a longer wavelength and lower frequency than visible light.
Why is it difficult to express the energy of a photon in joules per photon?
-It is difficult to express the energy of a photon in joules per photon because the values are very small, making them cumbersome to work with. Therefore, other units are often used to express these energies.
What is the importance of understanding the relationship between wavelength, frequency, and energy in the context of spectroscopy?
-Understanding the relationship between wavelength, frequency, and energy is crucial in spectroscopy as it allows scientists to identify and analyze the molecular structures of substances by correlating their absorption or emission spectra with these properties of light.
How does the example calculation in the transcript demonstrate the wave-particle duality of light?
-The example calculation shows the wave-particle duality of light by discussing both the wave properties (wavelength and frequency) and the particle properties (energy per photon). It illustrates how these different aspects of light can be calculated and related to each other.
What is Planck's constant and how is it used in the context of the calculation?
-Planck's constant is a fundamental constant in quantum mechanics, denoted by 'h', with a value of 6.626 x 10^-34 Js. It is used in the calculation to convert the frequency of light into its corresponding energy per photon using the equation E = h * nu.
Outlines
🔬 Infrared Spectroscopy and Organic Molecules
This paragraph discusses the application of infrared spectroscopy in analyzing functional groups within organic molecules. The focus is on the CH stretch region, which is indicative of CH single bonds. The absorption at approximately 3000 wave numbers is highlighted, and the process of converting this value to wavelength, frequency, and energy per photon is explained. The example demonstrates how to calculate the wavelength from wave numbers, determine the frequency using the speed of light, and find the energy of a single photon using Planck's constant. The comparison of the calculated energy with that of visible light provides context for the significance of these values in the study of light properties.
Mindmap
Keywords
💡Infrared Spectroscopy
💡Wave Number
💡Wavelength
💡Frequency
💡Photon
💡Planck's Constant
💡CH Stretch Region
💡Organic Molecules
💡Energy
💡Functional Groups
💡Light
Highlights
The discussion focuses on properties of light and its applications in infrared spectroscopy. (Start Time: 0s)
Infrared spectroscopy is used to analyze functional groups within organic molecules. (Start Time: 10s)
The CH stretch region is commonly examined in infrared spectroscopy. (Start Time: 20s)
Absorption at around 3,000 wave numbers is indicative of CH single bonds. (Start Time: 30s)
The example calculation involves converting wave numbers to wavelength, frequency, and energy per photon. (Start Time: 40s)
Wave number is defined as one over the wavelength. (Start Time: 50s)
The wavelength corresponding to 3,000 wave numbers is calculated to be 3.33 micrometers. (Start Time: 1m)
Frequency is determined by the equation c equals lambda times nu, where c is the speed of light. (Start Time: 1m 30s)
Frequency is calculated to be 8.99 x 10^13 cycles per second (hertz) for the 3,000 wave number CH stretch. (Start Time: 2m)
The energy per photon is found using Planck's constant multiplied by the frequency. (Start Time: 2m 30s)
The calculated energy per photon for the 3,000 wave number CH stretch is 5.96 x 10^-20 joules. (Start Time: 3m)
The example illustrates the conversion between different representations of light properties. (Start Time: 3m 30s)
The energy content per photon is small when compared to macroscopic scales. (Start Time: 4m)
Visible light has an energy of approximately 10^-19 joules per photon. (Start Time: 4m 30s)
The method allows for understanding the energy in a single photon, despite its small magnitude. (Start Time: 4m 45s)
The process demonstrates the interconnectivity of wavelength, frequency, and energy in the context of light. (Start Time: 5m)
Transcripts
Browse More Related Video
Chapter 18: Properties of Light | CHM 214 | 150
Electromagnetic Spectrum - Basic Introduction
Visible Light Spectrum Explained - Wavelength Range / Color Chart Diagram - Chemistry

Electromagnetic Spectrum Explained - Gamma X rays Microwaves Infrared Radio Waves UV Visble Light

Photons

How to Find the Wavelength, Frequency, Energy and Photons | Study Chemistry With Us
5.0 / 5 (0 votes)
Thanks for rating: