Electromagnetic Spectrum - Basic Introduction
TLDRThis educational video explores the electromagnetic spectrum, explaining how all types of electromagnetic waves travel at the speed of light and are distinguished by their wavelength, frequency, and energy. It emphasizes the relationship between these properties, noting that as wavelength increases towards radio waves, frequency and energy increase towards gamma rays. The script also covers how to calculate photon energy using Planck's constant and provides practice problems to illustrate these concepts, including determining the longest wavelength and highest frequency among different types of radiation, and calculating the frequency and energy of a UVA photon.
Takeaways
- 🌌 The electromagnetic spectrum includes various types of electromagnetic radiation, all traveling at the speed of light (3 x 10^8 meters per second).
- 🔄 The speed of light for an electromagnetic wave is the product of its wavelength and frequency.
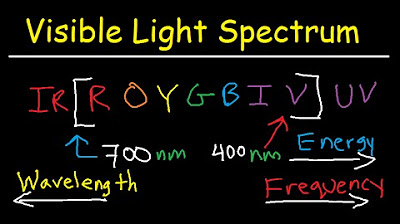
- 📏 As one moves from left to right across the spectrum, wavelength increases (from gamma rays to radio waves), and frequency decreases.
- 🔝 Conversely, as one moves from right to left, frequency increases (from radio waves to gamma rays), and so does the energy of the radiation.
- ⚡ Radio waves have the longest wavelength and the lowest frequency, while gamma rays have the shortest wavelength and the highest frequency.
- 🌈 Ultraviolet radiation has a higher frequency and more energy than visible light, and X-rays have a higher frequency than ultraviolet radiation.
- 💡 The energy of a photon is calculated using the equation: Energy = Planck's constant * Frequency, with Planck's constant being 6.626 x 10^-34 joule-seconds.
- 🔑 There's a direct proportionality between the frequency of a photon and its energy; as frequency increases, so does the energy.
- 📚 To determine the longest wavelength among different types of electromagnetic radiation, one should consider the position from left to right on the spectrum.
- 🔬 To find the highest frequency, one should look at the radiation types closest to the gamma rays end of the spectrum.
- 🔢 Given a wavelength, the frequency of a photon can be calculated using the formula: Frequency = Speed of light / Wavelength.
- ⚖️ The energy of a photon can be converted to electron volts using the conversion factor: 1 electron volt = 1.6 x 10^-19 joules.
Q & A
What is the speed of light in meters per second?
-The speed of light is 3 times 10 to the power of 8 meters per second.
What is the relationship between the speed of light and the wavelength and frequency of an electromagnetic wave?
-The speed of light of an electromagnetic wave is equal to the product of its wavelength and frequency.
Which type of electromagnetic radiation has the longest wavelength?
-Radio waves have the longest wavelength of all electromagnetic radiation in the spectrum.
What is the relationship between frequency and energy of a photon?
-The energy of a photon is directly proportional to its frequency, meaning as the frequency increases, so does the energy.
What is Planck's constant and how is it used in the equation for the energy of a photon?
-Planck's constant is 6.626 times 10 to the negative 34 joules times seconds. It is used in the equation for the energy of a photon as E = Planck's constant times the frequency.
Which electromagnetic radiation has the highest frequency?
-Gamma radiation has the highest frequency among all types of electromagnetic radiation.
What is the correct order of electromagnetic radiation from lowest to highest frequency?
-The order from lowest to highest frequency is: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma radiation.
Which color of visible light has the highest energy?
-Blue light has the highest energy among the colors of visible light listed in the script.
How can you calculate the frequency of a photon given its wavelength?
-You can calculate the frequency of a photon by dividing the speed of light by its wavelength.
What is the energy of a photon of UVA radiation with a wavelength of 200 nanometers?
-The energy of a photon of UVA radiation with a wavelength of 200 nanometers is approximately 9.939 times 10 to the negative 19 joules.
How can you convert the energy of a photon from joules to electron volts?
-You can convert the energy from joules to electron volts by dividing the energy in joules by 1.6 times 10 to the negative 19 joules, which is the value of one electron volt.
Outlines
🌌 Electromagnetic Spectrum Overview
This paragraph introduces the concept of the electromagnetic spectrum, explaining that all electromagnetic waves travel at the speed of light, which is 3 x 10^8 meters per second. It emphasizes the relationship between wavelength, frequency, and energy, noting that as wavelength increases towards radio waves, frequency and energy decrease, and vice versa towards gamma rays. The paragraph also explains Planck's equation, which relates photon energy to frequency, and introduces practice problems to compare different forms of electromagnetic radiation based on their wavelength, frequency, and energy.
🔬 Practice Problems on Electromagnetic Radiation
The second paragraph delves into practice problems related to the electromagnetic spectrum. It discusses how to determine which type of radiation has the longest wavelength and highest frequency from given options, using the knowledge that radio waves have the longest wavelengths and gamma rays have the highest frequencies and energies. The paragraph also covers the energy content of different colors of visible light, highlighting that blue light has more energy than red, yellow, or green light. Furthermore, it provides a step-by-step calculation for determining the frequency and energy of a UVA photon with a given wavelength, including the conversion of energy from joules to electron volts, to illustrate the photon's energy equivalence to an electron with a certain voltage.
Mindmap
Keywords
💡Electromagnetic Spectrum
💡Photons
💡Speed of Light
💡Wavelength
💡Frequency
💡Energy
💡Planck's Constant
💡Radio Waves
💡Gamma Rays
💡Visible Light
💡Electron Volts
Highlights
The electromagnetic spectrum includes various types of electromagnetic radiation carried by photons.
All electromagnetic waves travel at the speed of light, which is 3 x 10^8 meters per second.
The speed of light is equal to the product of wavelength and frequency of an electromagnetic wave.
As you move towards radio waves in the spectrum, the wavelength increases.
Radio waves have the longest wavelength of all electromagnetic radiation.
Frequency increases as you move towards the left of the spectrum, with gamma radiation having the highest frequencies.
Energy of electromagnetic radiation increases with frequency, with gamma rays having the most energy.
The energy of a photon is calculated using Planck's constant times the frequency.
Planck's constant is 6.626 x 10^-34 joules seconds.
The energy of a photon is directly proportional to its frequency.
To determine the longest wavelength, one should look towards the right of the spectrum.
Microwaves have the longest wavelength among the given options in the practice problem.
X-rays have the highest frequency among the listed electromagnetic radiation types.
Among visible light colors, blue light has the highest energy.
The frequency of a photon can be calculated using the speed of light divided by its wavelength.
A photon's energy can be found by multiplying Planck's constant with its frequency.
The energy of a photon can also be expressed in electron volts, with 1 eV equal to 1.6 x 10^-19 joules.
A UVA photon with a wavelength of 200 nanometers has an energy equivalent to 6.2 electron volts.
Transcripts
Browse More Related Video

Electromagnetic Spectrum Explained - Gamma X rays Microwaves Infrared Radio Waves UV Visble Light
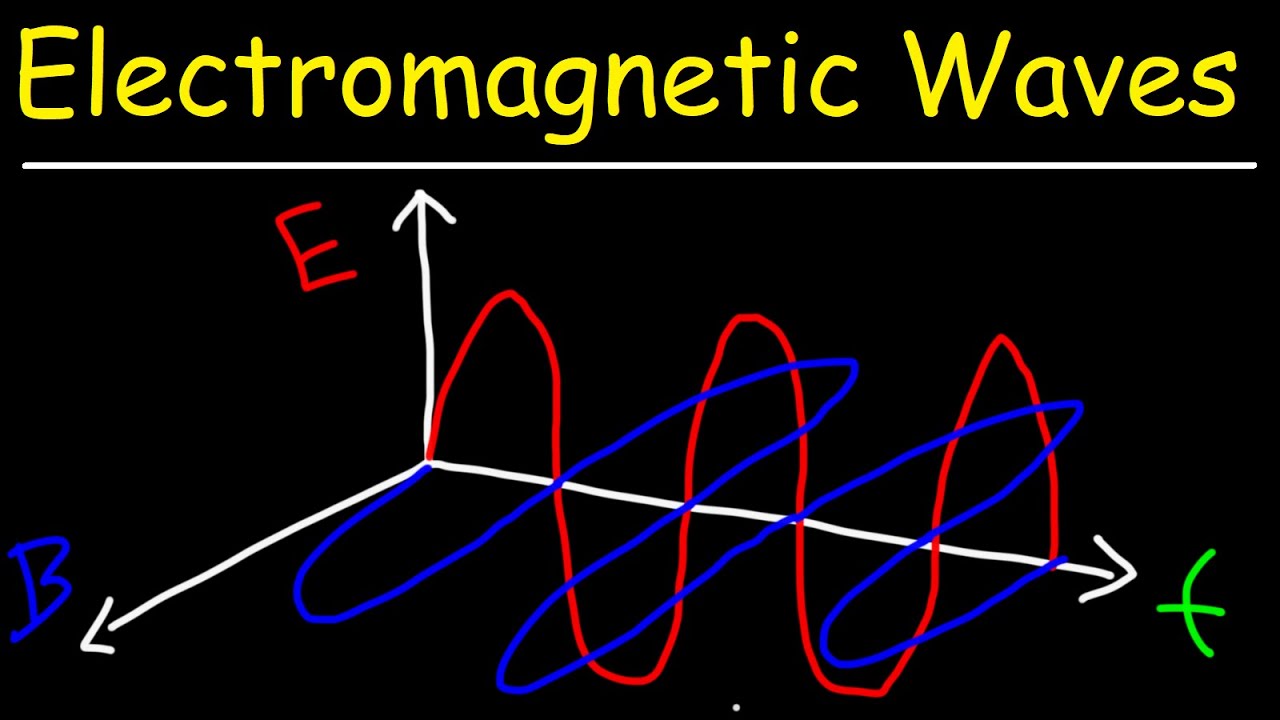
Electromagnetic Waves
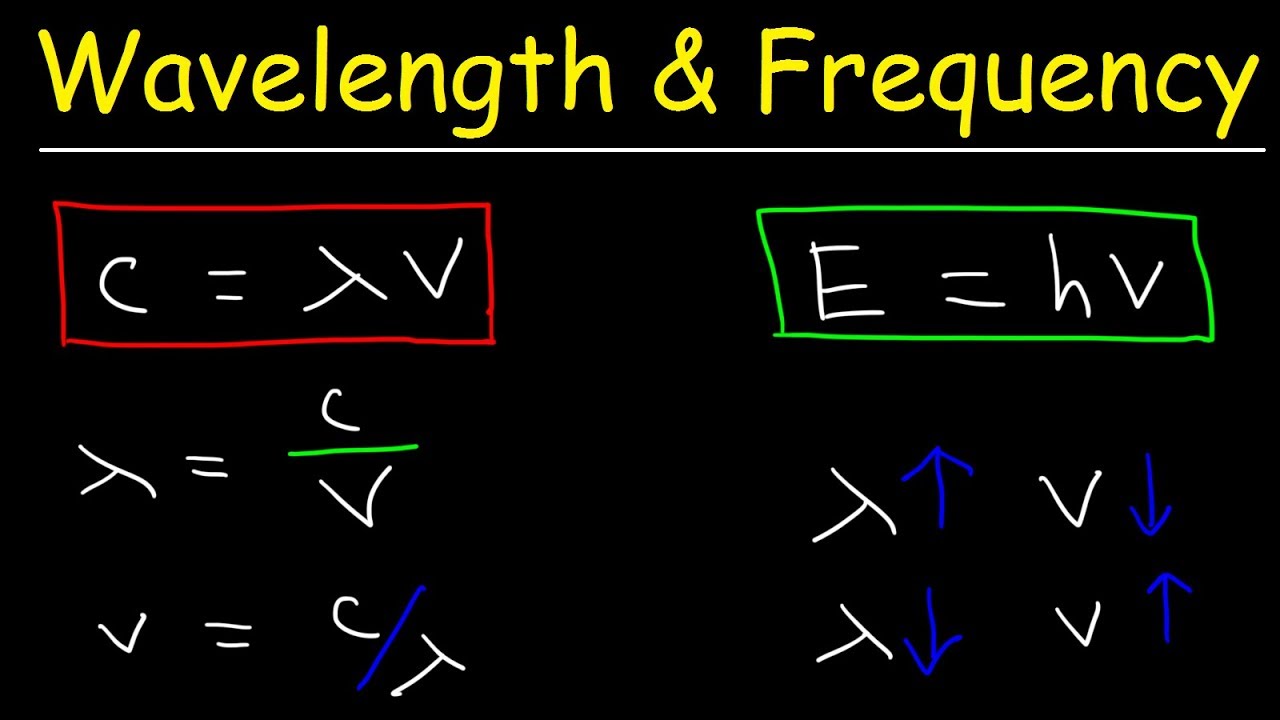
Speed of Light, Frequency, and Wavelength Calculations - Chemistry Practice Problems
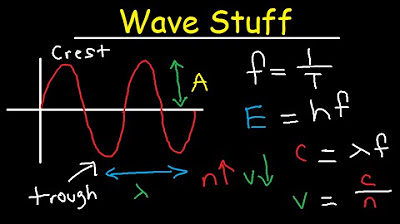
Wavelength, Frequency, Energy, Speed, Amplitude, Period Equations & Formulas - Chemistry & Physics
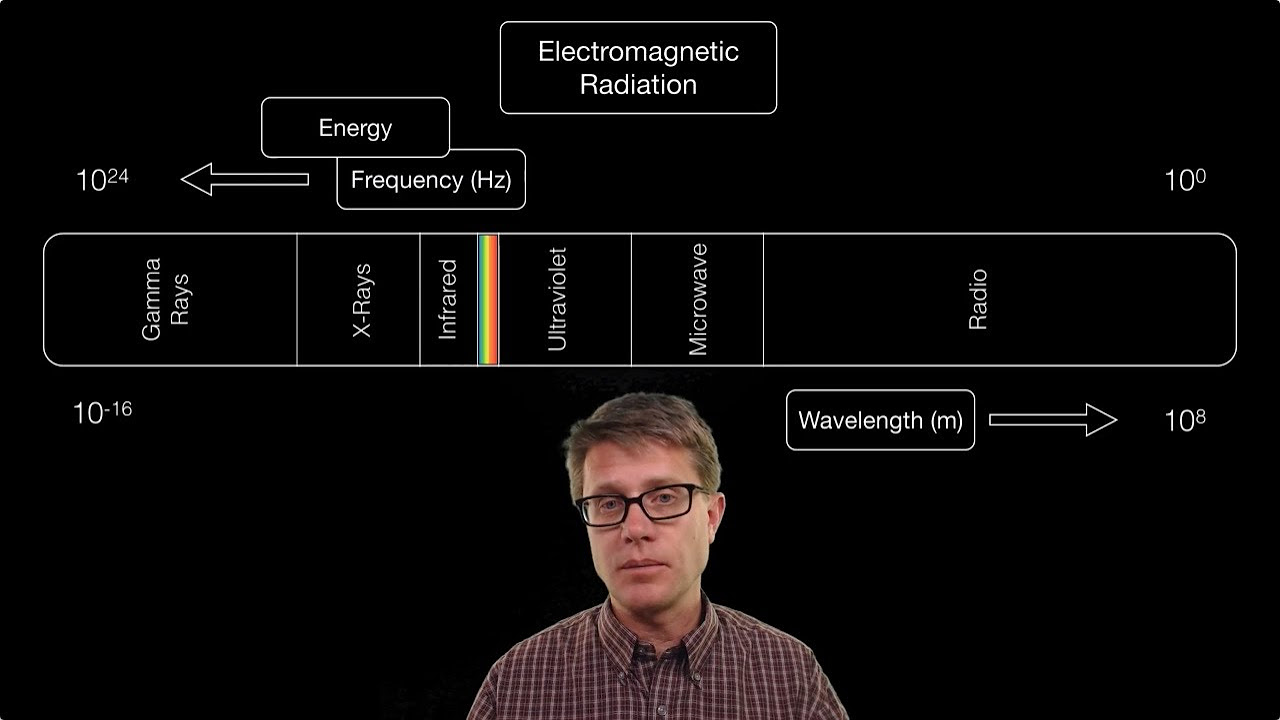
Electromagnetic Radiation
Visible Light Spectrum Explained - Wavelength Range / Color Chart Diagram - Chemistry
5.0 / 5 (0 votes)
Thanks for rating: