How to Find the Wavelength, Frequency, Energy and Photons | Study Chemistry With Us
TLDRThe video transcript focuses on chemistry concepts, specifically the relationship between wavelength and frequency, and the calculation of energy and number of photons. It introduces a custom study plan to help viewers understand and memorize key formulas, such as Planck's constant and the speed of light, and their applications in determining frequency, energy, and photon count. The transcript emphasizes the importance of unit conversion and precision in calculations, providing examples and explanations to clarify the concepts.
Takeaways
- 📚 The video is part of a series aimed at helping a fellow YouTuber, Melissa Lucy, and others, pass chemistry by providing a custom study plan with various resources.
- 🔍 The main focus of the video is on understanding and applying different formulas related to wavelength, frequency, energy, and the number of photons.
- 🔄 The relationship between wavelength and frequency is inversely proportional, which is a crucial concept to grasp for solving related problems.
- 🧠 Memorizing key formulas such as the one relating wavelength and frequency, and the one involving Planck's constant, energy, and the speed of light is emphasized for problem-solving.
- 🌟 The video provides a step-by-step guide on how to use these formulas, including the importance of unit conversion, especially from nanometers to meters.
- 📐 An example is given on how to calculate the energy of x-rays with a specific wavelength, highlighting the process of applying the formula and converting units.
- 📊 The video also discusses the significance of significant figures in calculations, and how to determine the correct number of significant figures to retain.
- 🌐 The concept of different units for the speed of light (C) is touched upon, noting that it can be either 2.998 or 3, depending on the context.
- 📱 Another example is provided to calculate the wavelength of an FM radio wave given its frequency, demonstrating the rearrangement of formulas and unit conversion.
- 💡 The video concludes with a problem involving the calculation of the number of photons in a flash of green light, given its wavelength and total energy.
- 🎓 Additional help and resources are available on Melissa's website for those who need further assistance in their chemistry studies.
Q & A
What is the relationship between wavelength and frequency in the context of the video?
-The relationship between wavelength and frequency is that they are inversely proportional to each other. This means that as the wavelength increases, the frequency decreases, and vice versa.
What is the main formula discussed in the video to relate wavelength and frequency?
-The main formula discussed in the video is e = h * c / λ, where e represents energy, h is Planck's constant, c is the speed of light, and λ (lambda) is the wavelength.
What is the significance of Planck's constant (H) in the energy formula?
-Planck's constant (H) is a fundamental constant in quantum mechanics that relates the energy of a photon to its frequency. It is used in the energy formula to calculate the energy of individual photons based on their wavelength.
How does the speed of light (C) affect the energy calculation of photons?
-The speed of light (C) is a critical factor in the energy calculation of photons as it is part of the formula that determines the energy based on the wavelength. A higher speed of light would result in different energy values for the same wavelength.
What is the importance of converting units when calculating the energy of photons?
-Converting units is crucial in ensuring that the calculations are accurate and that the final result is expressed in the correct units. For example, wavelengths need to be converted from nanometers to meters, and energy from kilojoules to joules, to match the units used in the formulas.
Why is it necessary to know the energy of a single photon before calculating the total number of photons in a given energy of light?
-Knowing the energy of a single photon is necessary because it allows you to determine the total number of photons in a larger energy value. This is done by dividing the total energy by the energy of a single photon, which gives the number of photons.
What is the significance of Avogadro's number in the context of calculating the energy of one mole of photons?
-Avogadro's number is used to convert the energy from joules to joules per mole, which is the unit required when calculating the energy of one mole of photons. It multiplies the energy of a single photon by the number of photons in one mole.
How does the concept of significant figures (sig figs) apply to the calculations in the video?
-Significant figures are important in maintaining the precision of the calculations. The number of significant figures retained in the final answer should match the number of significant figures in the given data, ensuring the accuracy of the results.
What is the role of the metric system in the video's calculations?
-The metric system is essential for converting units such as from nanometers to meters, which is necessary for the calculations involving wavelengths and energy. Correct unit conversion ensures that the formulas are applied accurately.
How does the video emphasize the importance of memorizing formulas in chemistry?
-The video emphasizes that memorizing formulas is crucial for understanding the relationships between different quantities in chemistry, such as wavelength, frequency, and energy. It allows for quick and accurate problem-solving and is necessary for successful learning and application in chemistry.
What is the purpose of the custom study plan mentioned in the video?
-The custom study plan is designed to help learners, like fellow YouTuber Melissa Lucy, pass their chemistry course. It includes several videos and resources aimed at reducing stress and helping students graduate faster by providing structured learning materials.
Outlines
📚 Introducing the Study Plan for Chemistry
This paragraph introduces a custom study plan designed by the speaker to help a fellow YouTuber, Melissa Lucy, and others pass chemistry. The study plan includes multiple resources such as videos, which can be accessed through a link in the description. The main focus of this segment is on understanding the relationship between wavelength and frequency, highlighting their inverse proportionality. The speaker emphasizes the importance of memorizing key formulas, which will be crucial for problem-solving and stress reduction during the study of chemistry.
🧪 Calculating Energy of X-Rays with Given Wavelength
In this paragraph, the speaker guides through a practical example of calculating the energy of x-rays using a given wavelength of 15.0 nanometers. The process involves understanding the relationship between wavelength and energy, and applying the formula E=hc/λ. The speaker also explains the necessity of unit conversion from nanometers to meters and emphasizes the importance of using correct units for scientific calculations. The example serves to illustrate the application of the formula and the significance of unit consistency in obtaining accurate results.
🌐 Converting FM Radio Wave Frequency to Wavelength
This section focuses on converting the frequency of an FM radio wave to its corresponding wavelength. The speaker provides the frequency of 102.5 megahertz and explains the need to convert this frequency into seconds to the negative first. The process involves understanding the relationship between frequency and wavelength, and using the formula c=λf. The speaker also discusses the importance of knowing the correct constants and units, and the potential variations in the speed of light value depending on the instructor's preference.
🌟 Energy Calculation for a Mole of Photons
The speaker discusses the concept of calculating the energy of one mole of photons, given a specific wavelength. The process involves converting the wavelength from nanometers to meters, applying the energy formula E=hc/λ, and then adjusting for the mole concept by dividing by Avogadro's number. The speaker emphasizes the importance of unit conversion and the significance of Avogadro's number in relating individual particles to moles. The example serves to illustrate the application of these concepts in chemistry and physics.
💡 Determining the Number of Photons in a Flash of Light
This paragraph focuses on calculating the number of photons contained in a flash of green light, given the wavelength and total energy. The speaker explains the two-step process of first finding the energy of individual photons using the formula E=hc/λ, and then determining the total number of photons by dividing the total energy by the energy per photon. The example highlights the importance of unit consistency and the application of fundamental physics formulas in real-world scenarios.
🎓 Conclusion and Additional Resources
In the concluding paragraph, the speaker wraps up the session by summarizing the key points discussed and encourages viewers to seek additional help if needed. The speaker promotes their website, MelissaMiracle.com, as a resource for further assistance and expresses hope that the viewers are enjoying the educational series. The speaker also reminds viewers to keep practicing with the formulas and concepts discussed to enhance their understanding of chemistry.
Mindmap
Keywords
💡Chemistry
💡Wavelength
💡Frequency
💡Formulas
💡Energy
💡Planck's Constant
💡Speed of Light
💡Photons
💡Conversion of Units
💡Significant Figures
Highlights
The relationship between wavelength and frequency is inversely proportional, which is a fundamental concept in understanding wave behavior.
The formula to relate wavelength (λ) and frequency (f) is given by the constant factor of the speed of light (c) and Planck's constant (h), expressed as c = λf.
Memorizing the key formulas is crucial for problem-solving in chemistry, especially those involving the calculation of frequency and energy.
The energy of a photon (E) can be calculated using the formula E = hν, where h is Planck's constant and ν is the frequency of the photon.
When given the wavelength, one can determine the energy of a photon using the relationship between wavelength, Planck's constant, and the speed of light.
Unit conversion is essential in chemistry problems, such as converting nanometers to meters to ensure compatibility with the formula's requirements.
The speed of light (c) can be expressed in different units, such as meters per second or centimeters per second, depending on the context.
Significant figures (sig figs) play a vital role in calculations, and one must be mindful of how many sig figs to retain based on the given data and the final result.
When calculating the energy of x-rays with a given wavelength, it's important to apply the correct formula and convert units accurately.
For problems involving the number of photons, one must first determine the energy of individual photons before calculating the total number.
The concept of one mole of photons is introduced, which involves understanding Avogadro's number and its application in chemistry.
When given a specific wavelength and total energy, one can calculate the number of photons present using the appropriate formulas and unit conversions.
The importance of unit consistency is emphasized when performing calculations, as incorrect units can lead to errors in the results.
The video provides a practical approach to solving chemistry problems, focusing on the application of formulas, unit conversions, and significant figures.
The series aims to help viewers pass chemistry by offering custom study plans and resources that can aid in stress reduction and faster graduation.
The video emphasizes the importance of understanding the basics of wave behavior and energy calculation as a foundation for more complex chemistry topics.
Transcripts
Browse More Related Video
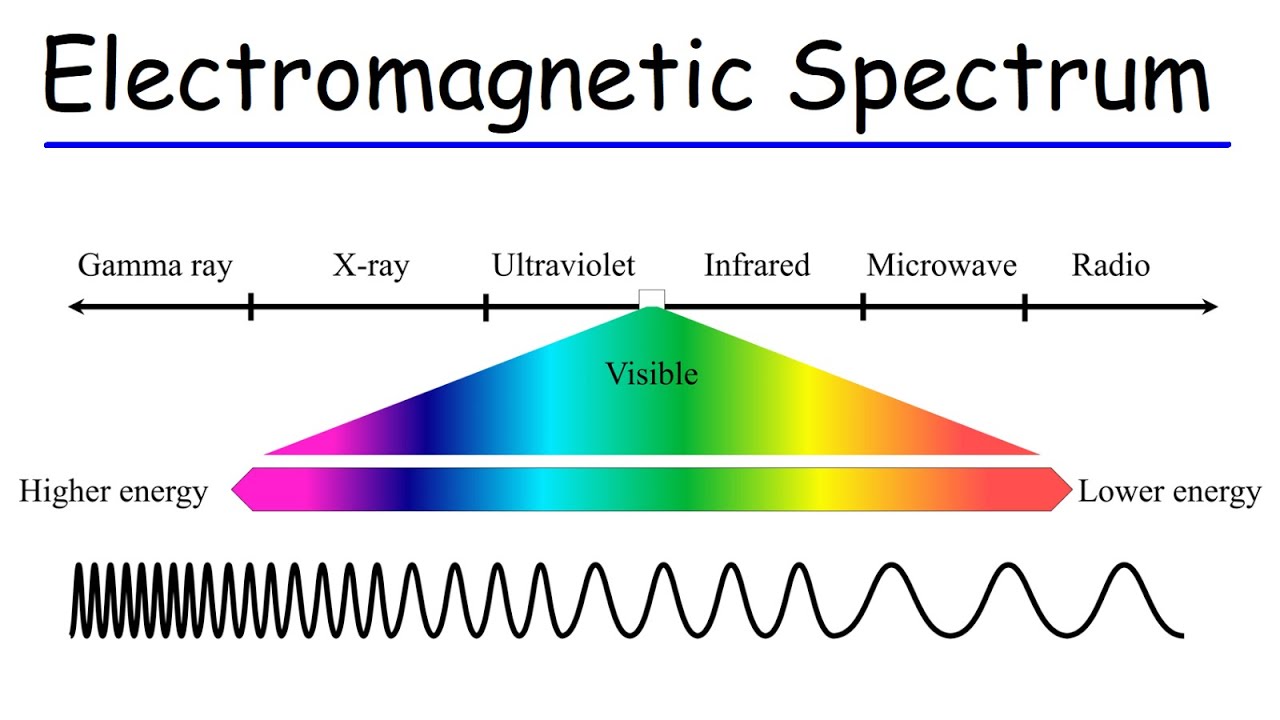
Electromagnetic Spectrum - Basic Introduction
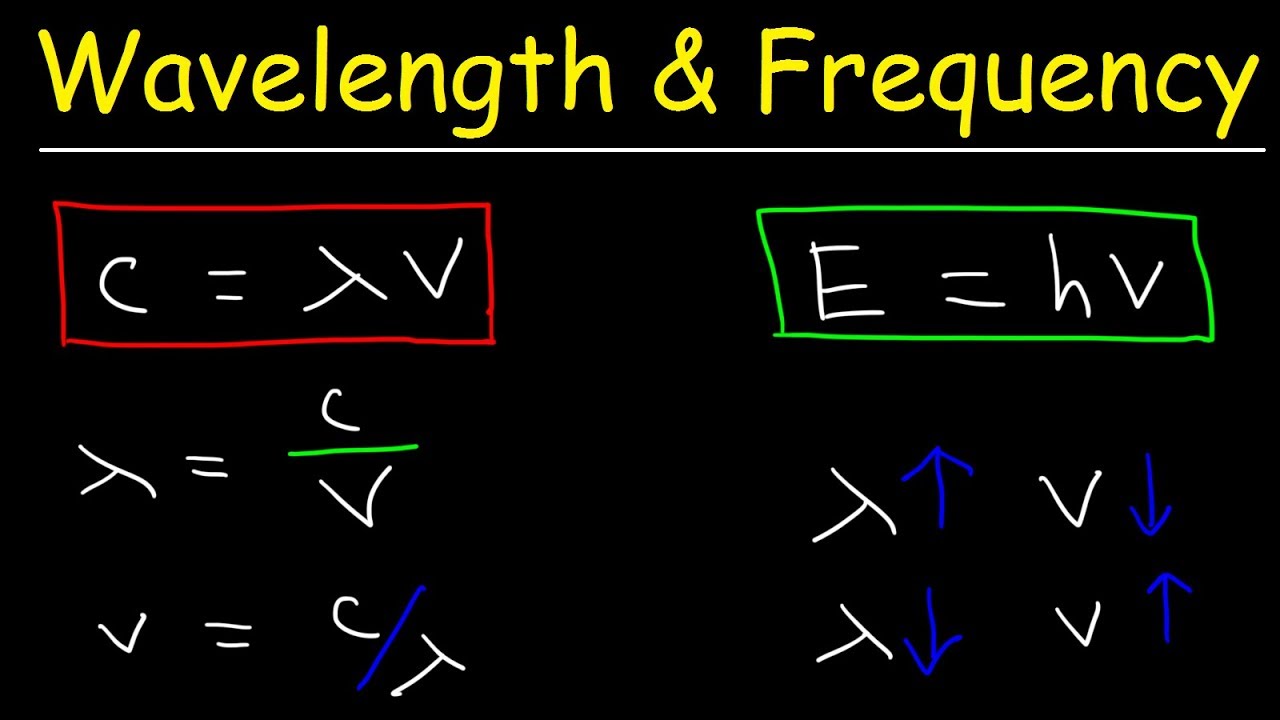
Speed of Light, Frequency, and Wavelength Calculations - Chemistry Practice Problems
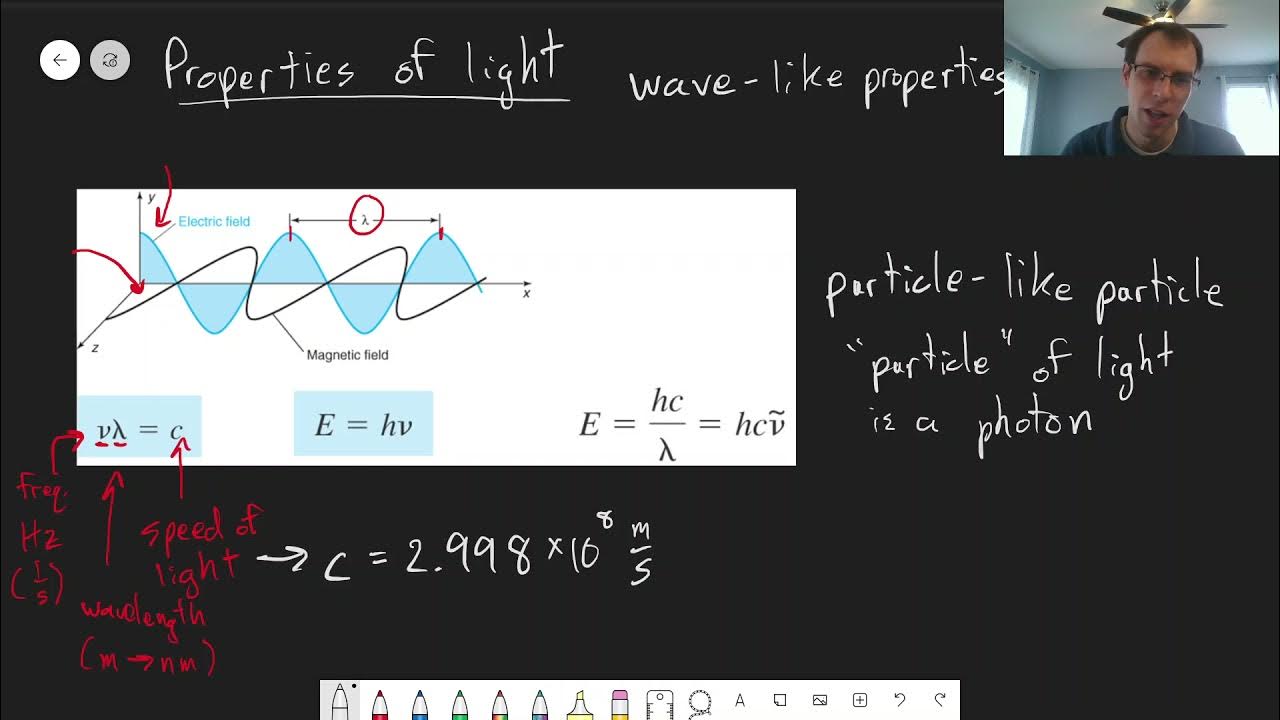
Chapter 18: Properties of Light | CHM 214 | 150

Electromagnetic Spectrum Explained - Gamma X rays Microwaves Infrared Radio Waves UV Visble Light
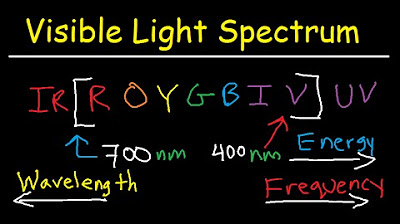
Visible Light Spectrum Explained - Wavelength Range / Color Chart Diagram - Chemistry

Chapter 18: Example Unit Conversions with Light | CHM 214 | 152
5.0 / 5 (0 votes)
Thanks for rating: