Lecture 3 Atomic Structure
TLDRThis video script delves into the atomic structure, explaining the fundamental particles that constitute an atom: electrons, protons, and neutrons, highlighting their differences in mass and charge. It also covers Dalton's atomic theory, which includes the indivisibility and indestructibility of atoms, and their role in chemical reactions to form molecules. The script points out the evolution of understanding from Dalton's theory to the discovery of subatomic particles.
Takeaways
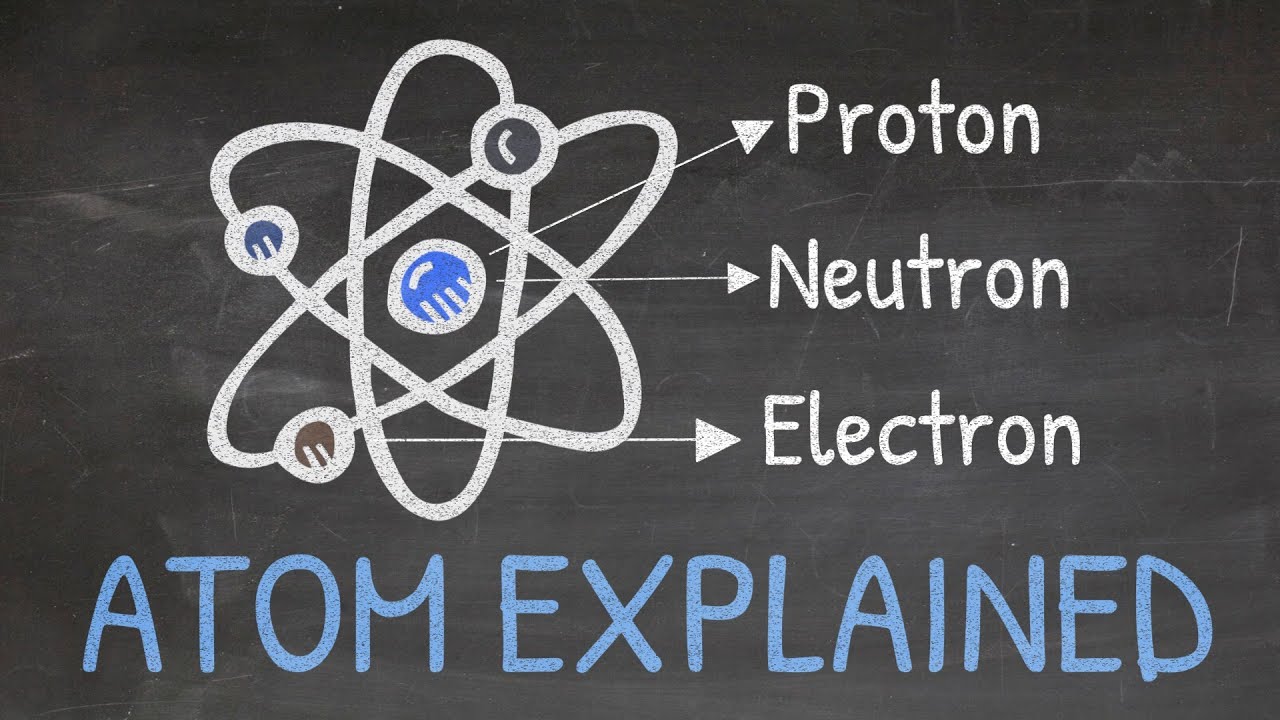
- 🌐 The atom is the fundamental unit of matter and consists of three subatomic particles: electrons, protons, and neutrons.
- ⚡ Electrons have a negative charge of -1.602 x 10^-19 Coulombs and a very small mass of 9.1 x 10^-31 kilograms.
- ➕ Protons carry a positive charge of +1.602 x 10^-19 Coulombs and have a mass of 1.6726 x 10^-27 kilograms.
- 🔴 Neutrons are neutral with no charge and have a mass nearly equal to that of protons.
- 🌟 The mass of an electron is significantly less than that of a proton or a neutron, which is why the mass of an atom is primarily determined by the mass of protons and neutrons.
- 📚 Dalton's atomic theory was a significant early model describing the composition and properties of atoms.
- 🔍 Dalton proposed that each element is made up of extremely small, identical particles called atoms.
- 🔄 Atoms of the same element are identical, but atoms of different elements are not.
- 🚫 Dalton's theory initially considered atoms to be indivisible and structureless, which was later disproved with the discovery of subatomic particles.
- ♻️ Atoms are indestructible; they cannot be created or destroyed in chemical reactions, adhering to the law of conservation of mass.
- 🧪 In chemical reactions, atoms of different elements combine to form molecules, as illustrated by the reaction X + Y forming compound XY.
Q & A
What are the three fundamental particles that make up an atom?
-The three fundamental particles that make up an atom are electrons, protons, and neutrons.
What is the charge of an electron in terms of magnitude and sign?
-The charge of an electron is -1.602 x 10^-19 coulombs, indicating it has a negative charge.
What is the mass of an electron in kilograms?
-The mass of an electron is approximately 9.109 x 10^-31 kilograms.
What is the charge of a proton in terms of magnitude and sign?
-The charge of a proton is +1.602 x 10^-19 coulombs, indicating it has a positive charge.
What is the mass of a proton in kilograms?
-The mass of a proton is approximately 1.6726 x 10^-27 kilograms.
What is the charge of a neutron?
-A neutron has a charge of zero, meaning it is electrically neutral.
How does the mass of an electron compare to the mass of a proton and a neutron?
-The mass of an electron is significantly less than the mass of a proton and a neutron.
What is Dalton's atomic theory?
-Dalton's atomic theory is a foundational concept in chemistry that states each element is composed of extremely small, identical particles called atoms, which have characteristic masses and are indestructible.
According to Dalton's theory, what are the characteristics of atoms of the same element?
-According to Dalton's theory, atoms of the same element are identical in mass and chemical properties.
What was the initial belief about atoms according to Dalton's theory?
-The initial belief according to Dalton's theory was that atoms were the ultimate particles with no structure.
How does Dalton's theory describe the indestructibility of atoms?
-Dalton's theory states that atoms are indestructible, meaning they cannot be created or destroyed in chemical reactions.
What is the role of atoms in chemical reactions according to Dalton's theory?
-According to Dalton's theory, atoms of different elements combine in chemical reactions to form molecules of compounds.
Outlines
🌌 Introduction to Atomic Structure
The script introduces the topic of atomic structure, focusing on the fundamental particles that constitute an atom: electrons, protons, and neutrons. It explains the differences in mass and charge among these particles, with electrons being the lightest and having a negative charge, protons having a positive charge and a greater mass, and neutrons being neutral with a mass similar to that of protons. The script also touches on Dalton's atomic theory, which includes the idea that atoms are indestructible and that chemical reactions involve the combination of atoms from different elements to form compounds.
Mindmap
Keywords
💡Atomic Structure
💡Subatomic Particles
💡Electron
💡Proton
💡Neutron
💡Dalton's Atomic Theory
💡Element
💡Chemical Reaction
💡Indestructible
💡Ultimate Particle
💡Molecule
Highlights
Introduction of the topic 'Atomic Structure' focusing on the structure of the atom and its constituent particles.
Atoms consist of three particles: electrons, protons, and neutrons, with distinct differences in mass and charge.
Electrons have a negative charge of -1.602 x 10^-19 coulombs and a mass of 9.1 x 10^-31 kilograms.
Protons have a positive charge of +1.602 x 10^-19 coulombs and a mass of 1.6726 x 10^-27 kilograms.
Neutrons are neutral with a mass nearly equal to that of a proton.
The mass of an electron is significantly less than that of a proton or a neutron.
The mass of an atom is primarily determined by the mass of protons and neutrons.
Dalton's atomic theory is introduced, outlining the fundamental principles of atomic structure.
Dalton's first postulate: Each element is composed of extremely small particles called atoms.
Dalton's second postulate: Atoms of a particular element are identical, differing from atoms of other elements.
Dalton's third postulate: Atoms of each element are ultimate particles with characteristic mass but no structure.
The discovery of electrons, protons, and neutrons contradicts Dalton's third postulate.
Dalton's fourth postulate: Atoms are indestructible and cannot be created or destroyed.
Dalton's fifth postulate: Atoms of an element participate in chemical reactions to form molecules.
Chemical reactions involve the combination of atoms from different elements to form compounds.
The historical significance of Dalton's atomic theory and its evolution with the discovery of subatomic particles.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: