11.2 Dilutions and Solution Stoichiometry | High School Chemistry
TLDRThis chemistry lesson focuses on dilutions and solution stoichiometry, essential for high school students. The instructor explains the concept of dilution using a relatable Kool-Aid example and then delves into the dilution equation, emphasizing the importance of consistent units. The second half of the lesson covers solution stoichiometry, illustrating how to calculate volumes of reactants in chemical reactions using molarity and mole ratios. Practical examples, such as the reaction between lead nitrate and hydrochloric acid, are provided to solidify the concepts, making complex topics more accessible.
Takeaways
- 😀 Dilution is the process of making a solute more dilute by adding more solvent or other substances that are not the solute.
- 📚 The key to dilution is that the amount of solute remains constant while the volume of the solution increases.
- 🧪 The dilution equation is m1v1 = m2v2, where m1 and m2 are the initial and final molarities, and v1 and v2 are the initial and final volumes.
- 🔍 The dilution equation can be used with any unit of concentration, not just molarity, as long as the units are consistent on both sides of the equation.
- 📉 When the volume of a solution increases, the concentration of the solute decreases proportionally.
- 🌡️ In the example given, 10 mL of 0.4 M NaCl is diluted to a total volume of 40 mL, resulting in a final concentration of 0.1 M.
- 🧪 Solution stoichiometry involves using molar ratios from balanced chemical equations to determine the volumes of solutions needed for complete reactions.
- 📚 The molarity of a solution (moles of solute per liter of solution) is crucial in calculating the volumes needed for stoichiometric reactions.
- 🌐 In the example of lead nitrate reacting with hydrochloric acid, the stoichiometric ratio is 1:2, meaning 2 moles of HCl are needed for every mole of lead nitrate.
- 📉 For the neutralization reaction between phosphoric acid and sodium hydroxide, the stoichiometric ratio is 1:3, indicating that three moles of NaOH are needed for every mole of H3PO4.
Q & A
What is a dilution in chemistry?
-A dilution is the process of making a solute go from a more concentrated to a less concentrated state, typically by adding more solvent.
What is the key concept to remember when performing a dilution?
-The key concept is that the moles of solute remain constant before and after the dilution. Only the amount of solvent changes.
How do you calculate the moles of solute in a solution?
-The moles of solute can be calculated using the formula: moles = molarity × volume (in liters).
What is the classic dilution equation?
-The classic dilution equation is M1V1 = M2V2, where M stands for molarity and V stands for volume. This equation shows the relationship between the initial and final concentrations and volumes.
Can the dilution equation be used with units other than molarity?
-Yes, the dilution equation can be used with any unit of concentration, such as mass percent, molality, or mole fractions, as long as the units are consistent on both sides of the equation.
What must you ensure when using the dilution equation with different units of volume?
-You must ensure that the units of volume are the same on both sides of the equation, whether they are milliliters, liters, or any other unit.
How do you interpret V2 in the dilution equation?
-V2 represents the total final volume of the solution, which includes both the original amount of solution and the added solvent.
How does increasing the volume of a solution affect its concentration?
-Increasing the volume of a solution decreases its concentration. For example, quadrupling the volume reduces the concentration to one-fourth of the original.
What is solution stoichiometry?
-Solution stoichiometry involves using mole ratios from a balanced chemical equation to relate quantities of reactants and products in solutions.
How do you determine the volume of a reactant needed in a stoichiometry problem?
-To determine the volume of a reactant needed, you calculate the moles of reactant required based on the mole ratio from the balanced equation, and then use the molarity of the reactant to find the volume.
Outlines
📘 Introduction to Dilutions and Solution Stoichiometry
In this lesson, the focus is on dilutions and solution stoichiometry. The lesson is part of a high school chemistry series, with new lessons released weekly. It begins with a definition of dilution and an example involving Kool-Aid to illustrate the concept of making a solution less concentrated by adding more solvent.
🔢 The Dilution Equation
The lesson explains that during dilution, the number of moles of solute remains the same while the volume increases, leading to decreased concentration. The dilution equation (m1v1 = m2v2) is derived from the molarity formula. The importance of using consistent units on both sides of the equation is emphasized.
🧪 Applying the Dilution Equation
An example problem is solved using the dilution equation. The problem involves diluting 10 milliliters of 0.4 M NaCl to a total volume of 40 milliliters. The calculation demonstrates that the final concentration is 0.1 M. Another example shows how to calculate the final concentration when 40 milliliters of water is added to 10 milliliters of 0.4 M NaCl.
⚖️ Introduction to Solution Stoichiometry
The lesson transitions to solution stoichiometry, emphasizing mole-to-mole ratios in chemical reactions. An example problem involves reacting 662.4 grams of lead(II) nitrate with 6 M HCl. The stoichiometric calculation determines the volume of HCl required for complete reaction, illustrating the concept of using molarity to relate moles to volume.
🔄 Solving Stoichiometry Problems
Another example problem involves an acid-base neutralization reaction between phosphoric acid and sodium hydroxide. The stoichiometry and molarity concepts are used to calculate the volume of NaOH needed to react completely with a given volume and concentration of phosphoric acid. The process involves determining moles, using mole ratios, and converting moles to volume.
Mindmap
Keywords
💡Dilution
💡Molarity
💡Stoichiometry
💡Concentration Units
💡Dilution Equation
💡Solution Stoichiometry
💡Mole Ratio
💡Neutralization Reaction
💡Molar Mass
💡Dimensional Analysis
Highlights
Introduction to dilutions and solution stoichiometry as part of a high school chemistry lesson series.
Definition of a dilution: making a solute go from more concentrated to less concentrated by adding solvent.
Example of dilution with Kool-Aid: Starting with a highly concentrated mixture and diluting it by adding water.
Key concept: The moles of solute remain constant during a dilution, only the solvent amount changes.
Derivation of the classic dilution equation: M1V1 = M2V2, where M is molarity and V is volume.
Explanation that the dilution equation can be used with any unit of concentration, as long as units are consistent on both sides.
Importance of using the same units of concentration and volume on both sides of the dilution equation.
Clarification of total volume (V2) in dilution: It's the combined volume of the original solution and the added solvent.
Practical example: Diluting 10 mL of 0.4 M NaCl to a total volume of 40 mL, resulting in a final concentration of 0.1 M.
Variation in problem: Adding 40 mL of water to 10 mL of 0.4 M NaCl gives a total volume of 50 mL, resulting in a concentration of 0.08 M.
Introduction to solution stoichiometry: Relating moles of reactants to moles of products in a chemical reaction.
Example of stoichiometry with a precipitation reaction: Lead nitrate and hydrochloric acid reacting to form lead chloride.
Calculating the volume of HCl needed to react with a given amount of lead nitrate, using molar mass and molarity concepts.
Using dimensional analysis and stoichiometry to solve for the volume of NaOH needed to react with a given volume of phosphoric acid.
Final example: Determining that 150 mL of 0.10 M NaOH is required to completely react with 25 mL of 0.20 M H3PO4.
Encouragement to like, share, and subscribe for more lessons, and information about additional study resources on chadsprep.com.
Transcripts
Browse More Related Video

Concentration and Molarity: The Key to Chemical Solutions

Chemistry | Stoichiometry | How to calculate percentage yield
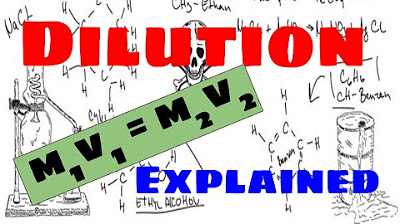
Dilution Explained
Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations Chemistry
Stoichiometry Tutorial: Step by Step Video + review problems explained | Crash Chemistry Academy

Molarity, Solution Stoichiometry and Dilution Problem
5.0 / 5 (0 votes)
Thanks for rating: