Other Periodic Table Trends
TLDRThis video script delves into ionization energy, electronegativity, and metallic nature, exploring their trends across the periodic table. It explains how elements like cesium readily give up electrons due to low ionization energy, while helium resists due to high energy required. The script also introduces the concept of second ionization energy, highlighting unexpected patterns like lithium's high second ionization energy post its low initial ionization energy. Electronegativity is attributed to atoms' tendencies to attract electrons in covalent bonds, with oxygen exemplifying high electronegativity. Metallic nature is associated with the willingness to lose electrons, increasing towards the bottom left of the periodic table. Lastly, atomic radius trends are discussed, noting that atoms expand down groups and contract across periods.
Takeaways
- 🔋 Ionization energy is the energy required to remove an electron from an atom, with cesium having a low ionization energy due to its large size and helium a high one due to its small size and strong electrostatic attraction.
- 🔑 The second ionization energy is the energy needed to remove an electron after the first has been removed, and elements with very low first ionization energy, like lithium, have a very high second ionization energy.
- 🌟 Electronegativity, introduced by Linus Pauling, is the measure of an atom's ability to attract shared electrons in a covalent bond, with oxygen being more electronegative than hydrogen.
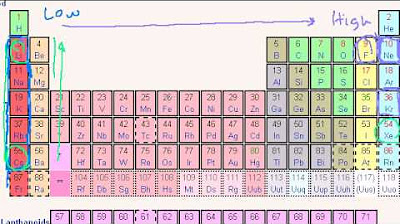
- ⚛️ Electronegativity increases towards the top right of the periodic table, where atoms have a stronger attraction for electrons, and decreases towards the bottom left, where atoms are more willing to give up electrons.
- 📉 Metallic nature is the tendency of an element to give away electrons, which increases towards the bottom left of the periodic table where atoms are larger and have weaker electrostatic forces.
- 📈 Atomic radius generally increases as you move down a group and decreases as you move from left to right within a period due to increasing nuclear charge pulling electrons inward.
- 🌐 The trend for ionization energy, electronegativity, and metallic nature is related to the atomic size and the strength of the electrostatic force between the nucleus and the electrons.
- 🔄 The concept of electron sharing in covalent bonds is not always equal, leading to differences in electronegativity between bonding atoms.
- 💡 Electronegativity differences in covalent bonds can be visualized by considering the time spent by electrons around each atom, with more electronegative atoms 'hogging' electrons more.
- 🛑 Noble gases are not discussed in terms of electronegativity because they are inert and do not typically form covalent bonds due to their satisfied electron configurations.
- 🔄 Trends in atomic properties such as ionization energy, electronegativity, and metallic nature are influenced by an atom's position in the periodic table, reflecting its structure and behavior.
Q & A
What is ionization energy and how does it vary across the periodic table?
-Ionization energy is the energy required to remove an electron from an atom. It generally decreases as you move from right to left across a period and increases as you move down a group in the periodic table.
Why does cesium have a low ionization energy?
-Cesium has a low ionization energy because it is a large atom with one extra electron in its sixth shell. It can easily give up this electron to achieve five complete shells, requiring very little energy to ionize.
What makes helium have a high ionization energy?
-Helium has a high ionization energy because it has a full first shell and is a very small atom with its electrons very close to the protons, resulting in a strong Coulomb force that makes it difficult to remove an electron.
Can you explain the concept of second ionization energy?
-Second ionization energy is the energy required to remove the second electron from an atom after the first one has been removed. It is often higher than the first ionization energy, especially for elements with low first ionization energies like lithium.
Why do elements with the lowest ionization energies have high second ionization energies?
-Elements with low ionization energies, like lithium, have high second ionization energies because once they lose one electron, their electron configuration becomes similar to a noble gas, making it very difficult to remove another electron.
What is electronegativity and who introduced this concept?
-Electronegativity is the measure of an atom's ability to attract electrons in a covalent bond. This concept was introduced by Linus Pauling.
How does electronegativity relate to the formation of covalent bonds?
-In a covalent bond, the sharing of electrons is not always equal. Atoms with higher electronegativity will attract the shared electrons more, creating a polar covalent bond.
Which elements are most electronegative and why?
-The most electronegative elements are the halogens, especially fluorine, because they are close to achieving a full valence shell and have a strong tendency to attract electrons.
What is the trend of electronegativity across the periodic table?
-Electronegativity increases as you move from left to right across a period and decreases as you move down a group in the periodic table.
What is the difference between metallic and non-metallic character in terms of electron affinity?
-Metallic character refers to the tendency of an element to give away electrons, while non-metallic character refers to the tendency to gain or share electrons. Elements with high metallic character are found on the left side and bottom of the periodic table, while non-metals are found on the top right.
How does atomic radius vary within a period and a group in the periodic table?
-Within a period, atomic radius decreases from left to right due to increasing nuclear charge pulling electrons closer. Within a group, atomic radius increases as you move down due to the addition of more electron shells.
Outlines
🔋 Ionization Energy and Second Ionization Energy
This paragraph discusses ionization energy, which is the energy needed to remove an electron from an atom. It explains the trend across the periodic table, with elements like cesium having low ionization energy due to their large size and desire to lose an electron to achieve a stable electron configuration. Conversely, helium, being small with a full first shell, has a high ionization energy. The concept of second ionization energy is introduced, which is the energy required to remove an additional electron after the first one has been removed. It's noted that elements with the lowest first ionization energy, like lithium, paradoxically have a very high second ionization energy because they achieve a stable configuration similar to helium after losing one electron, making it very difficult to remove another.
🔗 Electronegativity and Covalent Bonds
The paragraph delves into the concept of electronegativity, introduced by Linus Pauling, which refers to the tendency of atoms to attract electrons in a covalent bond. It explains that in covalent bonds, electrons are shared between atoms, but not equally; some atoms, like oxygen, are more electronegative and tend to 'hog' electrons. The trend of electronegativity across the periodic table is discussed, with the halogens, especially fluorine, being the most electronegative due to their strong desire to gain electrons to complete their valence shell. The paragraph also contrasts the electronegativity of elements with their metallic nature, highlighting the difference between atoms that readily give away electrons and those that are eager to gain them.
🌟 Metallic Nature and Atomic Radius Trends
This section explores the concept of metallic nature, which is associated with the willingness of an element to give away electrons. It is linked to properties like electrical conductivity, shininess, and malleability. The paragraph describes the trend of metallic nature across the periodic table, with elements on the bottom left, such as alkali metals, having a high metallic nature due to their large size and weaker hold on outer electrons. In contrast, elements on the top right, like the halogens, have a low metallic nature as they are more likely to gain electrons. The trend of atomic radius is also discussed, explaining that atomic size increases as you move down a group and decreases as you move across a period from left to right, due to the increasing nuclear charge pulling electrons closer.
📚 Summary of Chemical Properties and Trends
The final paragraph summarizes the key points discussed in the script, including ionization energy, electronegativity, metallic nature, and atomic radius. It emphasizes the importance of understanding these trends for predicting the chemical properties and behaviors of elements. The paragraph also hints at upcoming topics, such as bonding, which will be explored in future videos, suggesting a continuation of the educational journey through the periodic table and its implications for chemistry.
Mindmap
Keywords
💡Ionization Energy
💡Second Ionization Energy
💡Electronegativity
💡Covalent Bond
💡Metallic Nature
💡Atomic Radius
💡Electron Configuration
💡Coulomb Force
💡Periodic Table Trends
💡Linus Pauling
💡Halogens
Highlights
Ionization energy is the energy required to remove an electron and varies across the periodic table.
Cesium has a low ionization energy due to its large size and desire to lose an electron to achieve a stable electron configuration.
Helium has a high ionization energy because of its small size and strong coulomb force between electrons and protons.
Second ionization energy refers to the energy needed to remove the next electron after the first and can be higher than the first ionization energy.
Elements with high second ionization energies are often those with the lowest first ionization energies, such as lithium.
Lithium's electron configuration becomes stable like helium's after losing one electron, making the second ionization very difficult.
Electronegativity, introduced by Linus Pauling, measures an atom's ability to attract electrons in a covalent bond.
Oxygen is more electronegative than hydrogen, as it strongly attracts electrons to complete its valence shell.
Electronegativity increases towards the top right of the periodic table, where atoms are more likely to attract electrons.
Metallic nature is associated with an atom's willingness to give away electrons and is linked to properties like conductivity and malleability.
Metallic nature increases towards the bottom left of the periodic table, where atoms are larger and have weaker coulomb forces.
Atomic radius generally increases as you go down a group and decreases as you move across a period from left to right.
The atomic radius is influenced by the number of energy levels or shells an atom has, with more shells resulting in a larger atom.
The increase in protons in the nucleus as you move across a period pulls electrons inward, reducing the atomic size.
The trend in atomic radius is from the bottom right to the top left of the periodic table, with the largest atoms typically found in the lower periods.
The concept of electron sharing in covalent bonds and the unequal distribution of electrons based on electronegativity is crucial for understanding chemical bonding.
Electronegativity differences between atoms in a bond can lead to polar covalent bonds where electrons are more attracted to the more electronegative atom.
Understanding the trends in ionization energy, electronegativity, and metallic nature is essential for predicting chemical reactivity and bonding characteristics.
Transcripts
Browse More Related Video
Periodic Table Trends: Ionization Energy

Periodic Trends of the Periodic Table

Ionization Energy Electron Affinity Atomic Radius Ionic Radii Electronegativity Metallic Character

Periodic Trends - Atomic Radius, Electronegativity, Ionization Energy - Chemistry Series

Trends in the Periodic Table

How the MCAT Tests - Periodic Table Trends & Physics Correlates
5.0 / 5 (0 votes)
Thanks for rating: