Homotopic, Enantiotopic, Diastereotopic, and Heterotopic Protons
TLDRThis script delves into the nuances of proton relationships in chemistry, specifically within NMR spectroscopy. It defines homotopic, enantiotopic, diastereotopic, and heterotopic protons through illustrative examples, explaining how these relationships affect the interpretation of NMR spectra. The video clarifies that homotopic and enantiotopic protons share the same chemical shift, while diastereotopic and heterotopic protons exhibit different shifts, providing essential insights for chemists analyzing molecular structures.
Takeaways
- 🧪 The script discusses different types of proton relationships in chemistry, particularly in NMR spectroscopy.
- 🔍 Homotopic protons are those where replacing one with another element in a molecule results in the same molecule, just different conformations.
- 🌀 Enantiotopic protons are related to each other in a way that replacing them with a different element results in enantiomers, molecules with opposite stereochemistry.
- 🤝 Diastereotoic protons are associated with the generation of diastereomers when replaced, which are not mirror images but have at least one stereocenter inverted.
- 🏗️ Heterotopic protons are distinct in that replacing them with another element leads to structural isomers, completely different molecules.
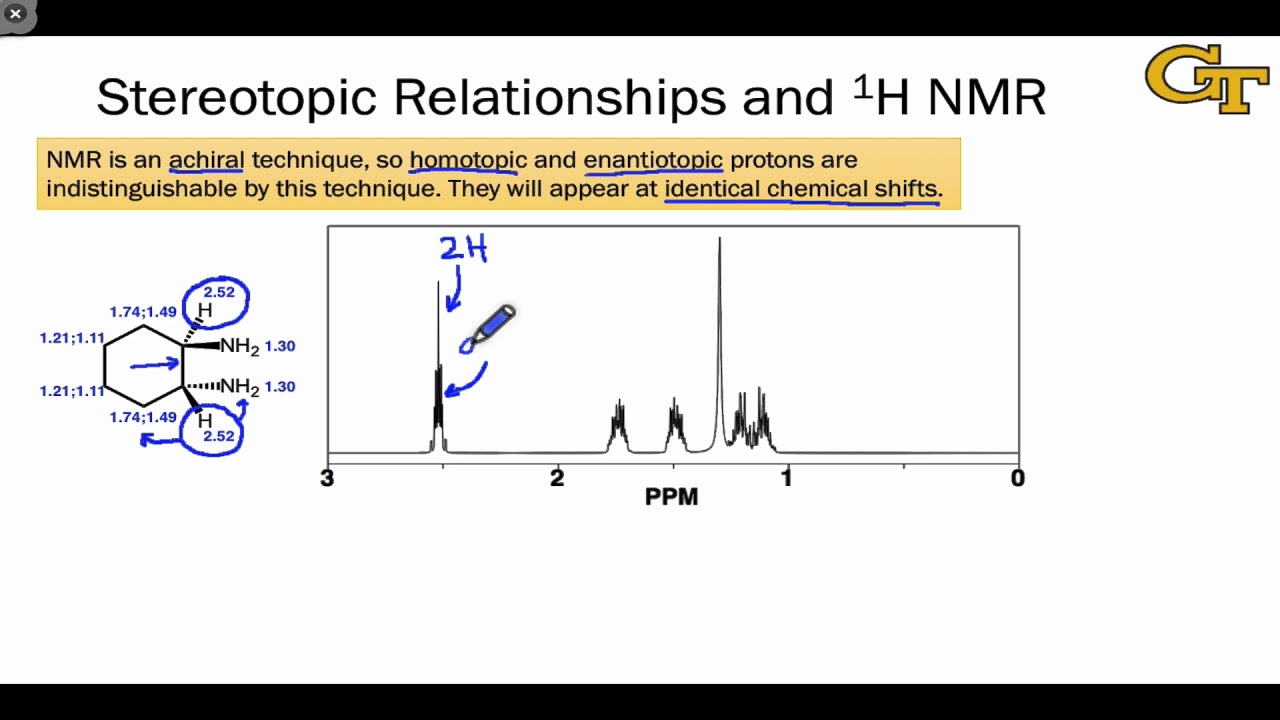
- 📊 In NMR spectroscopy, homotopic and enantiotopic protons exhibit the same chemical shift due to identical chemical environments.
- 📉 Diastereotoic and heterotopic protons, however, show different chemical shifts because they experience slightly different chemical environments.
- 🔑 Understanding these relationships is crucial for interpreting NMR spectra and distinguishing between types of protons based on their chemical shifts.
- 🌐 The script uses ethane, butane, a molecule with a hydroxyl group, and nitrobenzene as examples to illustrate the different types of proton relationships.
- 📚 The definitions of homotopic, enantiotopic, diastereotopic, and heterotopic are essential for understanding molecular structures and their NMR spectroscopic properties.
- 🧲 The presence of a chiral center influences the type of relationship protons can have, as seen with the example of a molecule with a six-membered ring and a bromo substituent.
Q & A
What are the different types of proton relationships discussed in the script?
-The script discusses homotopic, enantiotopic, diastereotopic, and heterotopic proton relationships.
How are homotopic protons defined in the context of NMR spectroscopy?
-Homotopic protons are those that, when replaced with another element in separate molecules, result in the same molecule or different conformations of the same molecule.
What is the significance of the term 'enantiotopic' in relation to protons?
-Enantiotropic protons are those that, when replaced with another element, generate enantiomers, which are non-superimposable mirror images of each other.
How do you determine if two protons have a diastereotopic relationship?
-Two protons have a diastereotopic relationship if replacing them with another element results in two different diastereomers, which have at least one but not all stereocenters inverted.
What is the definition of heterotopic protons?
-Heterotopic protons are those that, when replaced with another element, create structural isomers, which are different compounds with the same molecular formula but different connectivity of atoms.
Why are homotopic and enantiotopic protons important in NMR spectroscopy?
-Homotopic and enantiotopic protons are important in NMR spectroscopy because they all show the same chemical shift, indicating they are experiencing the same chemical environment.
How do diastereotopic and heterotopic protons differ in NMR spectroscopy?
-Diastereotopic and heterotopic protons differ in NMR spectroscopy as they show different chemical shifts due to experiencing slightly different chemical environments.
What is the difference between enantiomers and diastereomers as explained in the script?
-Enantiomers are mirror images of each other with opposite stereochemistry at all chiral centers, while diastereomers have at least one stereocenter with opposite configuration but are not mirror images.
How does the presence of a chiral center affect the relationship between protons?
-The presence of a chiral center affects the relationship between protons by potentially making them diastereotopic if replacing one of the protons with another element results in different diastereomers.
Can you give an example from the script where protons are considered heterotopic?
-An example from the script is nitrobenzene, where replacing two different hydrogens with fluorine results in structural isomers, indicating a heterotopic relationship.
What is the practical application of understanding these proton relationships in NMR spectroscopy?
-Understanding these proton relationships helps in interpreting NMR spectra by predicting which protons will show the same or different chemical shifts, aiding in the identification and characterization of molecules.
Outlines
🧪 Understanding Proton Relationships in NMR Spectroscopy
Professor Dave introduces the concept of different types of proton relationships in the context of NMR spectroscopy. He explains the terms homotopic, enantiotopic, diastereotopic, and heterotopic by using the example of ethane, butane, and other molecules. Homotopic protons are those that, when replaced by another atom, result in the same molecule. Enantiotopic protons, when substituted, create enantiomers. Diastereotop protons lead to the formation of diastereomers, which have at least one stereocenter inverted. Heterotopic protons, when substituted, result in structural isomers. The professor also discusses the implications of these relationships on the chemical shifts observed in NMR spectroscopy, noting that homotopic and enantiotopic protons have the same chemical shift, while diastereotopic and heterotopic protons exhibit different chemical shifts.
📊 NMR Chemical Shift Implications of Proton Relationships
This paragraph delves deeper into the implications of proton relationships on NMR spectroscopy. It emphasizes that homotopic and enantiotopic protons, due to their identical chemical environments, will show the same chemical shift in an NMR spectrum. In contrast, diastereotopic and heterotopic protons, which experience different chemical environments, will display distinct chemical shifts. The professor uses a complex molecule with a six-membered ring and a three-membered ring to illustrate how pairs of protons on the same carbon are diastereotopic due to the presence of a chiral center. This understanding is crucial for interpreting NMR spectra and distinguishing between different types of proton relationships.
Mindmap
Keywords
💡Homotopic
💡Enantiotopic
💡Diastereotopic
💡Heterotopic
💡NMR Spectroscopy
💡Chiral Center
💡Stereoisomers
💡Chemical Shift
💡Conformations
💡Stereocenters
💡Structural Isomers
Highlights
Introduction to different types of protons in chemistry and NMR spectroscopy.
Definition and explanation of homotopic protons using ethane as an example.
Demonstration of homotopic relationship through molecular manipulation with chlorine.
Clarification that homotopic protons result in the same molecule upon substitution.
Introduction to enantiotopic protons using butane as an example.
Explanation of enantiotopic relationship leading to enantiomers upon substitution.
Demonstration of generating opposite stereochemistry with enantiotopic protons.
Introduction to diastereotopic protons with a hydroxyl group on a carbon.
Explanation of diastereotopic relationship resulting in diastereomers, not enantiomers.
Clarification of the difference between diastereotopic and enantiotopic protons.
Introduction to heterotopic protons using nitrobenzene as an example.
Explanation of heterotopic relationship leading to structural isomers upon substitution.
Clarification that heterotopic protons result in completely different molecules.
Importance of understanding chemical shift differences for NMR spectroscopy.
Chemical shift similarities for homotopic and enantiotopic protons.
Chemical shift differences for diastereotopic and heterotopic protons.
Practical example of analyzing proton relationships in a complex molecule.
Identification of diastereotopic relationships in the example molecule.
Summary of the ability to assign proton relationships to any pair of protons.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: