NMR Spectroscopy
TLDRThe video script by Professor Dave dives into the intricacies of Nuclear Magnetic Resonance (NMR) spectroscopy, a vital tool in modern synthetic chemistry for identifying molecules and their structures. The script explains that while Infrared (IR) spectroscopy can identify functional groups, NMR is crucial for understanding the complete molecular structure, including connectivity and stereochemistry. The focus is on proton NMR, which involves analyzing the interaction of light with protium nuclei under an external magnetic field. The script breaks down the process of interpreting NMR spectra into three key components: chemical shift, integration, and splitting. Chemical shift indicates the environment of a proton, integration reflects the number of equivalent protons, and splitting patterns reveal the presence of neighboring protons. The video uses the example of bromoethane to illustrate how these components can be used to assign resonances to specific protons in a molecule, providing a foundational understanding of NMR spectroscopy for students of organic chemistry.
Takeaways
- 🧪 **NMR Spectroscopy Importance**: NMR (Nuclear Magnetic Resonance) spectroscopy is crucial for modern synthetic chemistry, allowing chemists to identify the exact structure of molecules, including connectivity and stereochemistry.
- 🌟 **Different Forms of NMR**: The script focuses on proton NMR, which involves studying protium nuclei and their interactions with an external magnetic field and light.
- 📊 **Understanding NMR Data**: Three main pieces of data are gleaned from an NMR spectrum: chemical shift, integration, and splitting, which provide insights into the chemical environment, number of equivalent protons, and neighboring protons respectively.
- 🧲 **Chemical Shift**: Indicates the chemical environment of a proton, with downfield shifts being closer to electronegative atoms and upfield shifts being farther away.
- 📈 **Integration**: Reflects the number of chemically equivalent protons contributing to a peak, with the area under the curve representing this quantity.
- 📞 **Splitting Pattern**: Describes the number of smaller peaks a resonance is split into, based on neighboring protons, following the n + 1 rule.
- 🏷️ **Assigning Resonances**: To assign peaks in an NMR spectrum, one must consider the chemical shift, integration, and splitting pattern to match the protons to the correct resonances.
- 🛑 **Deshielding Effect**: The presence of electronegative elements near a proton causes a deshielding effect, pulling electron density away from the nucleus and resulting in a downfield shift.
- 🔍 **Reference Peak**: Tetramethyl silane (TMS) is used as a reference peak in NMR, with all other peaks measured in parts per million relative to TMS.
- 🔬 **Interpreting Spectra**: The process of interpreting an NMR spectrum involves correlating the observed peaks with the expected chemical shifts, integrations, and splitting patterns based on the molecule's structure.
- 🧬 **Molecular Structure Determination**: NMR is a powerful tool for determining the precise structure of complex molecules, such as xerantholide, which is essential for multistep synthetic chemistry.
- ✍️ **Assignment Practice**: Students often practice NMR spectrum assignment by matching protons to peaks based on the three key data points, using simple molecules like bromoethane to build understanding.
Q & A
What is the primary purpose of using NMR spectroscopy in modern synthetic chemistry?
-The primary purpose of using NMR spectroscopy in modern synthetic chemistry is to identify the exact structure of a molecule, including all of its connectivity and stereochemistry, which is essential for multistep synthesis of complex molecules.
How does NMR spectroscopy differ from IR spectroscopy in its interaction with matter?
-While both are spectroscopic techniques, NMR spectroscopy differs from IR spectroscopy in that it focuses on the interaction of light with certain atomic nuclei that exhibit nuclear spin, as opposed to IR spectroscopy which studies the interaction of light with functional groups in a molecule.
What are the three main pieces of data obtained from an NMR spectrum?
-The three main pieces of data obtained from an NMR spectrum are the chemical shift, the integration, and the splitting pattern. These provide information about the chemical environment of protons, the number of chemically equivalent protons, and the number of neighboring protons, respectively.
What is the significance of the chemical shift in an NMR spectrum?
-The chemical shift in an NMR spectrum indicates the location of a proton's signal on the spectrum, which is related to the chemical environment of that proton. It tells us whether the proton is closer to electronegative atoms (downfield) or farther away from them (upfield).
How does the integration value in an NMR spectrum provide information about the molecule?
-The integration value in an NMR spectrum represents the area under the curve of a peak, which is proportional to the number of protons that are generating that resonance. It helps to determine how many chemically equivalent protons are present in a given environment within the molecule.
What is the n + 1 rule in the context of splitting patterns observed in NMR spectroscopy?
-The n + 1 rule states that a resonance generated by a proton will be split into a series of smaller peaks by neighboring protons, with the number of peaks being equal to the number of neighboring protons plus one (n + 1). This helps in determining the number of neighboring protons for a given proton in the molecule.
How does the presence of an electronegative element affect the chemical shift of a proton?
-The presence of an electronegative element affects the chemical shift of a proton by causing a deshielding effect. This occurs because the electronegative atom withdraws electron density away from the proton, which results in the signal being shifted further downfield (toward lower frequencies).
What does a singlet peak in an NMR spectrum indicate about the protons generating it?
-A singlet peak in an NMR spectrum indicates that the protons generating it do not have any neighboring protons. This means that these protons are in a chemical environment where they are not split into multiple peaks due to the absence of neighboring protons.
How can the structure of a molecule be deduced from its NMR spectrum?
-The structure of a molecule can be deduced from its NMR spectrum by analyzing the chemical shifts, integration values, and splitting patterns of the peaks. These data points provide information about the chemical environment of protons, their quantity, and their interaction with neighboring protons, which collectively help to determine the molecular structure.
What is the role of tetramethyl silane (TMS) in an NMR spectrum?
-Tetramethyl silane (TMS) is used as a reference compound in NMR spectroscopy. It provides a reference peak that is not part of the actual spectrum being analyzed. The chemical shifts of all other peaks in the spectrum are reported in parts per million (ppm) relative to the TMS reference peak.
Why is it important to understand the spatial arrangement of protons when analyzing an NMR spectrum?
-Understanding the spatial arrangement of protons is important because it helps in determining which protons are chemically equivalent and how they interact with neighboring protons. This is crucial for interpreting the splitting patterns and integration values, which in turn are key to deducing the structure of the molecule.
Outlines
🔬 Introduction to NMR Spectroscopy
Professor Dave introduces nuclear magnetic resonance (NMR) spectroscopy as a tool for identifying the precise structure of molecules, including connectivity and stereochemistry. He explains that unlike IR spectroscopy, NMR can provide detailed information about the chemical environment of every proton in a molecule. The process involves subjecting a molecule to an external magnetic field and then irradiating it with light to gather spectroscopic data. The professor also mentions that they will focus on proton NMR and discusses the basic principles without delving too deeply into the physics.
📊 Understanding NMR Spectra
The video script explains how to interpret NMR spectra by focusing on three main pieces of data: chemical shift, integration, and splitting. Chemical shift indicates the chemical environment of a proton, with downfield signals being closer to electronegative atoms. Integration refers to the area under a peak, which corresponds to the number of chemically equivalent protons contributing to that resonance. Splitting patterns, governed by the n + 1 rule, reveal the number of neighboring protons and are used to identify the structure of the molecule. The script uses the example of bromoethane to illustrate how these three aspects of NMR spectra can be used to assign peaks to specific protons in a molecule.
🧠 Assigning Proton Resonances in NMR
The final paragraph discusses the process of assigning resonances to specific protons in a molecule using NMR spectra. It emphasizes the importance of understanding the number of protons, their chemical equivalence, and the influence of neighboring protons on the splitting patterns. The script outlines a method for making predictions about the expected chemical shift, integration, and splitting for each resonance, using a simple molecule as an example. It concludes by reiterating the three key aspects to consider when analyzing NMR spectra: chemical shift, integration, and splitting pattern.
Mindmap
Keywords
💡NMR Spectroscopy
💡Proton NMR
💡Chemical Shift
💡Integration
💡Splitting
💡Tetramethyl Silane (TMS)
💡Electronegativity
💡Deshielding Effect
💡Xerantholide
💡Bromoethane
💡n + 1 Rule
Highlights
NMR spectroscopy is a powerful tool for identifying functional groups and determining the exact structure of complex molecules.
Proton NMR is used to study the interaction of light with protium nuclei, providing detailed information about the chemical environment of each proton in a molecule.
An external magnetic field is applied to a molecule, and the resulting spectroscopic data reveals how light interacts with the compound.
The chemical shift in NMR indicates the chemical environment of a proton, with downfield signals being closer to electronegative atoms.
Integration in NMR spectra represents the area under the curve of a peak, which corresponds to the number of chemically equivalent protons.
Splitting in NMR spectra is caused by neighboring protons and follows the n + 1 rule, where n is the number of neighboring protons.
A singlet in NMR indicates no neighboring protons, a doublet indicates one, a triplet indicates two, and so on.
The structure of bromoethane is used as an example to demonstrate how to assign peaks in an NMR spectrum based on chemical shift, integration, and splitting.
Chemically equivalent protons on the same carbon are considered identical in their chemical environment for the purpose of NMR analysis.
The chemical shift can be used to predict the relative position of a peak on the spectrum, with upfield signals being farther from electronegative elements.
Integration can be used to determine the number of protons contributing to a peak, aiding in the structural analysis of a molecule.
Splitting patterns provide insight into the number of neighboring protons, which is crucial for understanding the connectivity within a molecule.
The assignment of resonances to protons in an NMR spectrum involves analyzing chemical shift, integration, and splitting to deduce the molecular structure.
A simple method for assigning NMR spectra involves identifying the number of protons, their chemical environment, and neighboring protons to match peaks with structural elements.
The use of a reference peak, such as TMS (tetramethyl silane), helps in calibrating the chemical shift values of other protons in the spectrum.
Consulting a table of chemical shifts for different functional groups can provide more precise ppm values for protons in an NMR spectrum.
The practical application of NMR spectroscopy is demonstrated through the step-by-step analysis and assignment of peaks for a given molecule.
Transcripts
Browse More Related Video

12.02 Carbon-13 NMR Spectroscopy
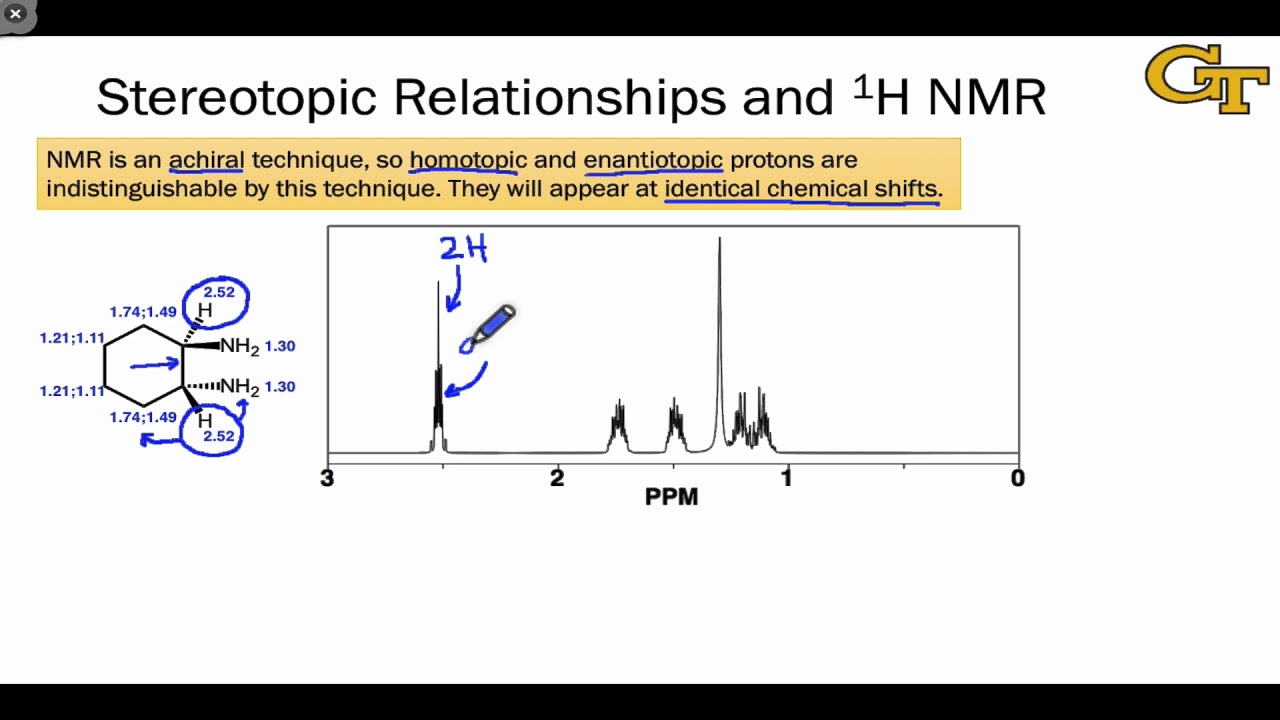
12.01 Stereotopic Relationships, Chemical Shift, and Coupling
How to Identify Molecules - Proton NMR: Crash Course Organic Chemistry #26

Shielding and Deshielding - H NMR Spectroscopy

Homotopic, Enantiotopic, Diastereotopic, and Heterotopic Protons

12.04 Two-dimensional NMR Spectroscopy
5.0 / 5 (0 votes)
Thanks for rating: