15.4 Homotopic vs Enantiotopic vs Diastereotopic | Organic Chemistry
TLDRThe provided transcript discusses the concept of stereochemistry in organic chemistry, specifically focusing on the relationship between hydrogen atoms (hydrants) in a molecule. The video explains three key terms: homotopic, enantiotopic, and diastereotopic, which describe the chemical equivalence and spatial arrangement of hydrogen atoms. Homotopic hydrogens are chemically equivalent and can be interconverted without changing the molecule's configuration. Enantiotopic hydrogens, when replaced with a unique label (e.g., the letter 'Z'), result in mirror-image molecules, known as enantiomers. Lastly, diastereotopic hydrogens lead to different signals in an H NMR spectrum, as they are not chemically equivalent and do not give rise to mirror-image molecules. The transcript uses examples to illustrate how to identify these relationships and their implications on the number of signals observed in an NMR spectrum, which is crucial for understanding molecular structure and reactivity.
Takeaways
- 🧪 **Homotopic Hydrogens**: Two hydrogens on the same carbon are considered homotopic if replacing one with a 'Z' and rotating the molecule 180 degrees makes it identical to the original.
- 🔍 **Enantiotopic Hydrogens**: If replacing a hydrogen on a 'CH2' with 'Z' creates a chiral center, and doing so with both hydrogens results in enantiomers (mirror images), the hydrogens are enantiotopic.
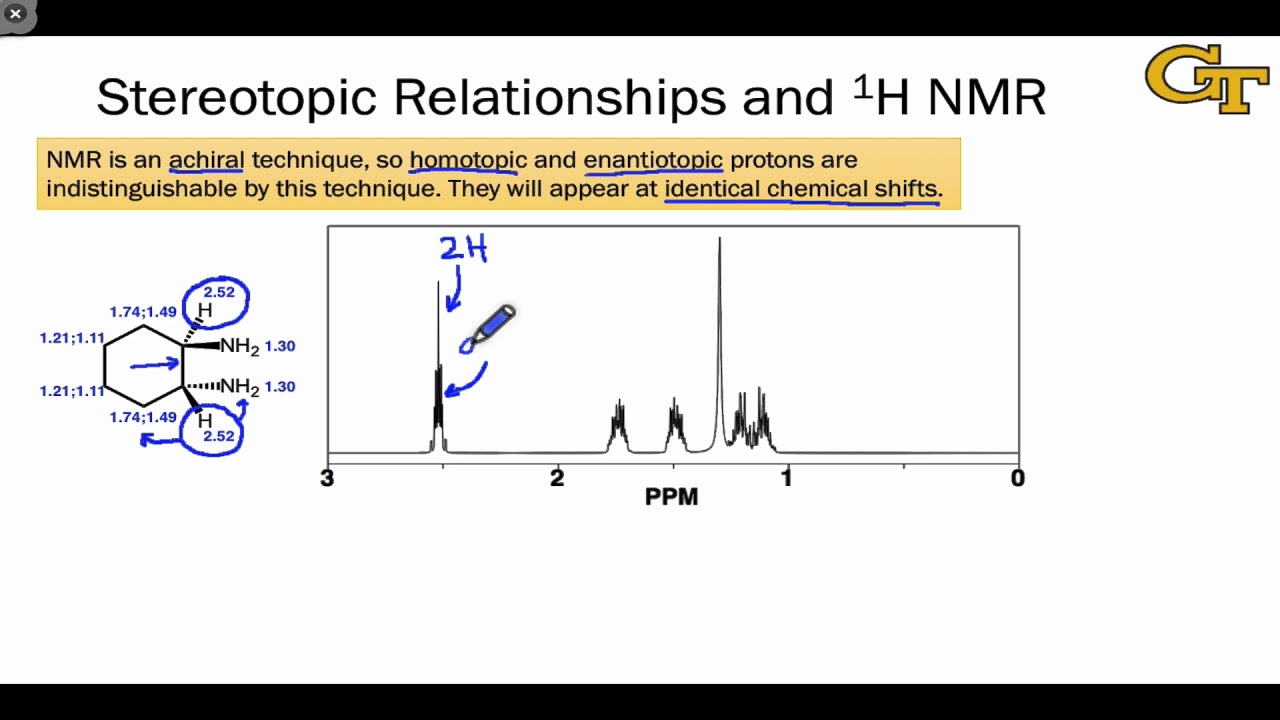
- 📶 **Chemical Equivalence**: Enantiotopic hydrogens are chemically equivalent and will give rise to the same signal in an H NMR spectrum.
- 🔬 **Diastereotopic Hydrogens**: When replacing hydrogens with 'Z' on a chiral center results in diastereomers (non-mirror image, non-identical stereoisomers), the hydrogens are diastereotopic.
- 🚫 **Distinct Signals**: Diasteretopic hydrogens are not chemically equivalent and will produce different signals in an H NMR spectrum.
- ⚖️ **Chiral Centers**: The presence of a chiral center in a molecule affects the relationship (homotopic, enantiotopic, or diastereotopic) of hydrogens on adjacent carbons.
- 🔄 **Free Rotation**: Hydrogens that are equivalent due to free rotation around a carbon-carbon single bond will also give a single signal in the H NMR spectrum.
- 🔀 **Symmetry Considerations**: Symmetry in a molecule can help determine the equivalence of hydrogen environments and the number of signals expected in the H NMR spectrum.
- 🧬 **Sequential Replacement**: The process of replacing hydrogens with 'Z' one at a time is crucial for determining the relationship between hydrogens and their equivalence or difference.
- 🔑 **Key to NMR Spectroscopy**: Understanding the relationships between hydrogens (homotopic, enantiotopic, diastereotopic) is key to interpreting H NMR spectra and determining the number of signals.
- 📈 **Signal Count**: The total number of unique signals in an H NMR spectrum can be predicted by analyzing the equivalence of hydrogen environments in a molecule.
Q & A
What is the term 'homotopic' referring to in the context of chemistry?
-Homotopic refers to hydrogen atoms that are in a position that can be interconverted by a plane of symmetry in a molecule, meaning they are chemically equivalent and will give rise to the same signal in an H NMR spectrum.
How do you determine if two hydrogens are homotopic?
-To determine if two hydrogens are homotopic, replace one hydrogen with the letter 'Z' and the other with the letter 'E'. If the resulting molecules are identical or can be made identical by a 180-degree rotation, the hydrogens are homotopic.
What is the difference between enantiotopic and diastereotopic hydrogens?
-Enantiotopic hydrogens, when replaced with 'Z', result in molecules that are mirror images (enantiomers) of each other, but not identical. Diastereotopic hydrogens, on the other hand, result in diastereomers, which are non-superimposable and non-mirror image stereoisomers.
Why are enantiotopic hydrogens considered chemically equivalent?
-Enantiotopic hydrogens are chemically equivalent because they give rise to the same signal in an H NMR spectrum, indicating they are in equivalent environments within the molecule.
How does the presence of a chiral center affect the determination of homotopic, enantiotopic, or diastereotopic hydrogens?
-The presence of a chiral center can change the relationship between hydrogens. If replacing a hydrogen with 'Z' creates a new chiral center, the hydrogens can be enantiotopic or diastereotopic. If no new chiral center is created, they are likely homotopic.
What is the significance of identifying hydrogens as homotopic, enantiotopic, or diastereotopic in NMR spectroscopy?
-Identifying the relationship between hydrogens is significant because it helps predict the number of signals that will be observed in an H NMR spectrum, which is crucial for understanding the molecule's structure and stereochemistry.
How does free rotation affect the equivalence of hydrogens in a molecule?
-Free rotation allows hydrogens that are not inherently equivalent due to their connectivity to become equivalent over time, as they can rotate around the carbon-carbon single bond, leading to the same chemical environment.
What is the relationship between the number of signals in an H NMR spectrum and the stereochemistry of a molecule?
-The number of signals in an H NMR spectrum corresponds to the number of different chemical environments for hydrogens in the molecule. Different stereochemical relationships like homotopic, enantiotopic, and diastereotopic result in different numbers of signals.
How can you tell if a molecule has a chiral center?
-A molecule has a chiral center if an atom is bonded to four different groups. If replacing a hydrogen with 'Z' results in an atom bonded to four different groups, then that atom is a chiral center.
What is the process of replacing hydrogens with 'Z' called in stereochemistry?
-The process of replacing hydrogens with 'Z' to determine the stereochemical relationship between them is known as the Cahn-Ingold-Prelog priority sequence rules or the 'Z/E' convention.
Why are diastereotopic hydrogens important in the context of chemical reactions?
-Diastereotopic hydrogens are important because they are not chemically equivalent and can react differently with reagents. This distinction is crucial for predicting the outcome of reactions and understanding the stereoselectivity of a molecule.
Can you provide an example where diastereotopic hydrogens result in different signals in an H NMR spectrum?
-Yes, in a molecule with a chiral center, the hydrogens on a CH2 group that is not symmetrical with respect to the chiral center will be diastereotopic. Replacing these hydrogens with 'Z' and observing the resulting molecules as diastereomers will lead to two different signals in the H NMR spectrum.
Outlines
🧪 Understanding Homotopic, Enantiotopic, and Diasteretopic Hydrogens
The first paragraph introduces the concept of homotopic hydrogens, which are chemically equivalent and can be identified by replacing one hydrogen with the letter 'Z' and observing if the resulting structures are identical upon a 180-degree rotation. The paragraph also explains enantiotopic hydrogens, which when replaced with 'Z', result in enantiomers—mirror images that are not superimposable. Lastly, the concept of diastereotopic hydrogens is introduced, which are not chemically equivalent and will give rise to different signals in an H NMR spectrum. The paragraph uses the example of a molecule with a chiral center to illustrate this.
📊 Signal Identification in H NMR Spectroscopy
The second paragraph delves into the implications of hydrogen types on H NMR spectrum signals. It explains that diastereotopic hydrogens, due to their non-equivalence, will produce two distinct signals in the spectrum. The paragraph also discusses how free rotation can lead to hydrogens being chemically equivalent, resulting in fewer signals. Several examples are provided to illustrate how to determine the relationship between hydrogens and predict the number of signals in an H NMR spectrum, emphasizing the importance of identifying chiral centers and the symmetry of molecules.
🧬 Complex Example of Diasteretopic Hydrogens
The third paragraph presents a challenging example involving the identification of diastereotopic hydrogens. It demonstrates that even in the absence of apparent chiral centers, the replacement of hydrogens with 'Z' can create a chiral center, thus making the hydrogens diastereotopic. The paragraph walks through the process of replacing hydrogens and analyzing the resulting molecular configurations to determine the relationship between the hydrogens. It concludes with the observation that in the given example, there would be four distinct signals in the H NMR spectrum due to the presence of diastereotopic hydrogens.
Mindmap
Keywords
💡Homotopic
💡Chemically Equivalent
💡Enantiotopic
💡Diastereotopic
💡Chiral Center
💡H NMR Spectrum
💡Free Rotation
💡Stereoisomers
💡Wedge and Dashed Representation
💡Signal
💡Molecular Rotation
Highlights
The concept of homotopic hydrogens is introduced, which are chemically equivalent and can be identified by replacing one hydrogen with the letter 'Z'.
Homotopic hydrogens are identical when rotated 180 degrees, allowing for perfect alignment.
The term 'enantiotopic' is explained, relating to hydrogens that, when replaced with 'Z', result in enantiomers, or mirror images.
Enantiotopic hydrogens are also chemically equivalent and will give rise to the same signal in H NMR spectrum.
Diastereotopic hydrogens are identified as not chemically equivalent and will result in different signals in the H NMR spectrum.
Diastereotopic hydrogens are associated with chiral centers and can be identified by replacing hydrogens with 'Z' and observing the resulting stereoisomer relationship.
The importance of identifying the number of signals in the H NMR spectrum based on the equivalence of hydrogens is discussed.
A method for determining if hydrogens on a CH2 group are homotopic, enantiotopic, or diastereotopic through 'Z' substitution is presented.
Examples are provided to illustrate the process of identifying hydrogen relationships and their impact on H NMR signals.
The impact of symmetry within a molecule on the equivalence of hydrogens and resulting NMR signals is explained.
A detailed example demonstrates how changing a hydrogen to 'Z' can unexpectedly create a chiral center, affecting the relationship of other hydrogens.
The concept that the presence of a chiral center can alter the equivalence of hydrogens in a molecule is explored.
A comprehensive approach to identifying diastereotopic hydrogens in complex molecules is outlined, emphasizing the need for careful analysis.
The significance of understanding hydrogen relationships for predicting the number of signals in an H NMR spectrum is highlighted.
Practical applications of 'Z' substitution in determining the chemical and spatial relationships of hydrogens in organic chemistry are discussed.
The transcript provides a thorough explanation of stereoisomerism concepts and their implications for hydrogen NMR signal analysis.
A step-by-step guide is given for students to follow when identifying and categorizing hydrogens as homotopic, enantiotopic, or diastereotopic.
Transcripts
Browse More Related Video
12.01 Stereotopic Relationships, Chemical Shift, and Coupling

15.3 The Number of Signals in Proton NMR | Organic Chemistry

Practice Problem: Types of Protons

Homotopic, Enantiotopic, Diastereotopic, and Heterotopic Protons

How To Determine The Number of Signals In a H NMR Spectrum

5.1 Overview of Isomers | Constitutional Isomers and Stereoisomers | Organic Chemistry
5.0 / 5 (0 votes)
Thanks for rating: